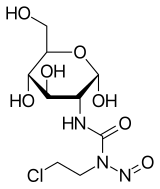
Chlorozotocin
Appearance
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C9H16ClN3O7 |
| Molar mass | 313.69 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Chlorozotocin is a nitrosourea. It is used for cancer therapy.[citation needed]
The International Agency for Research on Cancer concluded it was "probably carcinogenic" in 1990[1]
It is an analogue of streptozotocin.[2]
References
[edit]- ^ Humans, IARC Working Group on the Evaluation of Carcinogenic Risk to (1990). SUMMARY OF FINAL EVALUATIONS. International Agency for Research on Cancer.
- ^ Wolfe MM (2006). Therapy of digestive disorders. Elsevier Health Sciences. p. 477. ISBN 978-1-4160-0317-5.
