Volhard–Erdmann cyclization
Appearance
(Redirected from Volhard-Erdmann cyclization)
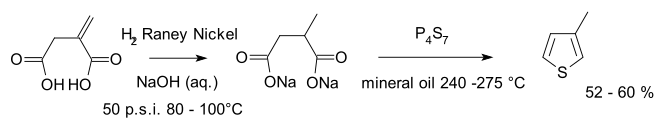
The Volhard–Erdmann cyclization is an organic synthesis of alkyl and aryl thiophenes by cyclization of disodium succinate or other 1,4-difunctional compounds (γ-oxo acids, 1,4-diketones, chloroacetyl-substituted esters) with phosphorus heptasulfide. The reaction is named after Jacob Volhard and Hugo Erdmann.[1]
An example is the synthesis of 3-methylthiophene starting from itaconic acid:[2]
References
[edit]- ^ Feldkamp, R. F.; Tullar, B. F. (1954). "3-Methylthiophene" (PDF). Organic Syntheses. 34: 73
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 4, p. 671. - ^ Volhard, J.; Erdmann, H. (1885). "Synthetische Darstellung von Thiophen". Chemische Berichte. 18 (1): 454–455. doi:10.1002/cber.18850180199.