Vince lactam

| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Azabicyclo[2.2.1]hept-5-en-3-one | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
| ||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
| ||
| |||
| |||
| Properties | |||
| C6H7NO | |||
| Molar mass | 109.128 g·mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H302, H317, H318 | |||
| P261, P264, P270, P272, P280, P301+P312, P302+P352, P305+P351+P338, P310, P321, P330, P333+P313, P363, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Vince lactam[1] is the commercial name given to the bicyclic molecule γ-lactam 2-azabicyclo[2.2.1]hept-5-en-3-one. This lactam is a versatile chemical intermediate used in organic and medicinal chemistry. It is used as a synthetic precursor for three drugs (approved or in clinical trials).[2][3] It is named after Robert Vince who has used the structural features of this molecule for the preparation of carbocyclic nucleosides.[4] Vince's work with this lactam eventually led to his synthesis of abacavir.[5][6][7] Peramivir synthesis is also dependent on Vince lactam starting material.
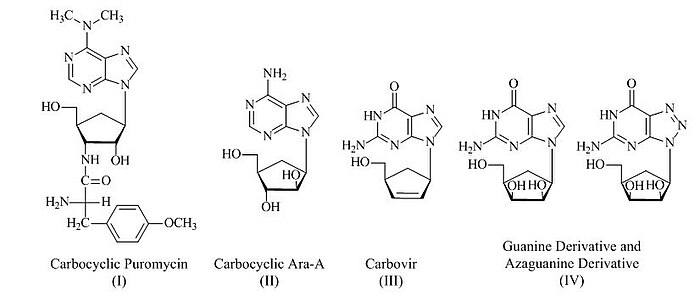
Vince lactam has been extensively used for the preparation of various carbocyclic nucleosides with medicinal applications in mind, including carbocyclic puromycin (I),[8] carbocyclic Ara-A (II),[9] carbovir (III)[10] and guanine as well as azaguanine carbocyclic derivatives (IV)[11]
Vince lactam is also an intermediate in the synthesis of various nucleoside analogs such as difluoro guanosine derivatives (V),[12][13] carbocyclic oxanosine and related derivatives (VI),[14] and precursors for azidocarbonucleosides (VII).[15] The lactam has found several applications in targeting an array of different diseased conditions by providing various non-nucleoside therapeutic molecules as well. Some well known examples include scaffolds for the preparation of glycosidase inhibitors (VIII)[16] and GABA-AT inhibitors (IX).[17]
References
[edit]- ^ Singh, R.; Vince, R. Chem. Rev. 2012, 112 (8), pp 4642–4686."2-Azabicyclo[2.2.1]hept-5-en-3-one: Chemical Profile of a Versatile Synthetic Building Block and its Impact on the Development of Therapeutics"
- ^ Rouhi, A. M. (July 14, 2003). "Simplifying Syntheses Is Always A Key Goal". C&EN. 80 (28): 40.
- ^ Holt-Tiffin, K. E. Chimica Oggi 2009, 27, 23-25.
- ^ "Robert Vince, Ph.D." Center for Drug Design, University of Minnesota.
- ^ Daluge, S.; Vince, R. J. Org. Chem. 1978, 43, 2311-2320.
- ^ Vince, R.; Hua, M. "Synthesis of carbovir and abacavir from a carbocyclic precursor" Current Protocols in nucleic acid chemistry Ed. Beaucage, S. L. 2006, Chapter 14 Unit 14.4. doi:10.1002/0471142700.nc1404s25.
- ^ Vince, R. "A brief history of the development of Ziagen" Chemtracts 2008, 21, 127-134.
- ^ Vince, R.; Daluge, S.; Brownell, J. J. Med. Chem. 1986, 29, 2400.
- ^ Daluge, S.; Vince, R. J. Org. Chem., 1978, 43, 2311-2320.
- ^ Vince, R.; Hua, M. J. Med. Chem. 1990, 33, 17.
- ^ Peterson, M. L.; Vince, R. J. Med. Chem. 1990, 33, 1214-1219.
- ^ Toyota, A.; Habutani, C.; Katagiri, N.; Kaneko, C. Tetrahedron Lett. 1994, 35, 5665-5668.
- ^ Toyota, A.; Aizawa, M.; Habutani, C.; Katagiri, N.; Kaneko, C. Tetrahedron 1995, 36, 8783-8798.
- ^ Saito, Y.; Nakamura, M.; Ohno, T.; Chaicharoenpong, C.; Ichikawa, E.; Yamamura, S.; Kato, K.; Umezawa, K. J. Antibiotics 2000, 53, 309-313.
- ^ Kiss, L.; Forro, E.; Sillanpaa, R.; Fulop, F. Synthesis 2010, 153-160.
- ^ Rommel, M.; Ernst, A.; Koert, U. Eur. J. Org. Chem. 2007, 4408-4430.
- ^ Mineno, T.; Miller, M,. J. J. Org. Chem. 2003, 68, 6591-6596.