Vapor: Difference between revisions
m Reverted edits by 14.96.123.195 (talk) to last revision by 213.60.23.78 (HG) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Other uses}} |
{{Other uses}} |
||
| ⚫ | etween gas and the critical point.|The vapour-liquid critical point in a pressure-temperature [[phase diagram]] is at the high-temperature extreme of the liquid-gas phase boundary. <small>(The dotted green line gives the anomalous behaviour of water.)</small>]]--[[Special:Contributions/86.96.226.18|86.96.226.18]] ([[User talk:86.96.226.18|talk]]) 16:54, 20 February 2013 (UTC) |
||
[[File:watervapor cup.jpg|thumb|right|Water condenses into visible droplets after evaporating from a cup of hot tea]] |
|||
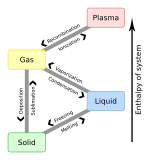
A '''vapour''' (British spelling) or '''vapor''' (see [[spelling differences]]) is a substance in the [[gas]] phase at a [[temperature]] lower than its [[critical temperature|critical point]].<ref>R. H. Petrucci, W. S. Harwood, and F. G. Herring, "General Chemistry", 8th edition (Prentice-Hall 2002), p.486.</ref> |
|||
This means that the vapour can be [[condensation|condensed]] to a [[liquid]] or to a [[solid]] by increasing its [[pressure]] without reducing the temperature. |
|||
For example, water has a critical temperature of 374 °C (647 K), which is the highest temperature at which liquid water can exist. In the [[Earth's atmosphere|atmosphere]] at ordinary temperatures, therefore, gaseous water (known as [[water vapor]]) will condense to liquid if its [[partial pressure]] is increased sufficiently. |
|||
A vapour may co-exist with a liquid (or solid). When this is true, the two phases will be in equilibrium, and the gas pressure will equal the equilibrium [[vapour pressure]] of the liquid (or solid).<ref>Petrucci et al. p.483</ref> |
|||
== Properties == |
|||
| ⚫ | |||
''Vapour'' refers to a gas phase at a temperature where the same substance can also exist in the [[liquid]] or [[solid]] state, below the [[critical temperature]] of the substance. (For example, water has a critical temperature of 374 °C (647 K), which is the highest temperature at which liquid water can exist.) If the vapour is in contact with a liquid or solid phase, the two phases will be in a state of [[thermodynamic equilibrium|equilibrium]]. The term ''gas'' refers to a compressible fluid phase. Fixed gases are gases for which no liquid or solid can form at the temperature of the gas, such as air at typical ambient temperatures. A liquid or solid does not have to boil to release a vapour. |
|||
Vapor is responsible for the familiar processes of [[cloud]] formation and [[condensation]]. It is commonly employed to carry out the physical processes of [[distillation]] and [[headspace technology|headspace extraction]] from a liquid sample prior to [[gas chromatography]]. |
|||
The constituent [[molecule]]s of a vapor possess vibrational, rotational, and translational motion. These motions are considered in the [[kinetic theory of gases]]. |
|||
==Vapour pressure== |
|||
{{Main|Vapour pressure}} |
|||
[[File:Binary_Boiling_Point_Diagram_new.svg|left|thumb|Liquid-Vapour Equilibrium]] |
[[File:Binary_Boiling_Point_Diagram_new.svg|left|thumb|Liquid-Vapour Equilibrium]] |
||
The [[vapour pressure]] is the equilibrium pressure from a liquid or a solid at a specific temperature. The equilibrium vapour pressure of a liquid or solid is not affected by the amount of contact with the liquid or solid interface. |
The [[vapour pressure]] is the equilibrium pressure from a liquid or a solid at a specific temperature. The equilibrium vapour pressure of a liquid or solid is not affected by the amount of contact with the liquid or solid interface. |
||
| Line 31: | Line 14: | ||
*[[Earth's atmosphere|Atmospheric]] [[water vapour]] is found near the earth's surface, and may condense into small liquid droplets and form meteorological phenomena such as [[fog]], [[mist]] and [[haar (fog)|haar]]. |
*[[Earth's atmosphere|Atmospheric]] [[water vapour]] is found near the earth's surface, and may condense into small liquid droplets and form meteorological phenomena such as [[fog]], [[mist]] and [[haar (fog)|haar]]. |
||
*[[Mercury-vapour lamp]]s and [[sodium vapour lamp]]s produce light from atoms in [[excited state]]s. |
*[[Mercury-vapour lamp]]s and [[sodium vapour lamp]]s produce light from atoms in [[excited state]]s. |
||
--[[Special:Contributions/86.96.226.18|86.96.226.18]] ([[User talk:86.96.226.18|talk]]) 16:54, 20 February 2013 (UTC) |
|||
==Measuring vapour== |
==Measuring vapour== |
||
Since it is in the |
Since it is in the g--[[Special:Contributions/86.96.226.18|86.96.226.18]] ([[User talk:86.96.226.18|talk]]) 16:54, 20 February 2013 (UTC)--[[Special:Contributions/86.96.226.18|86.96.226.18]] ([[User talk:86.96.226.18|talk]]) 16:54, 20 February 2013 (UTC)--[[Special:Contributions/86.96.226.18|86.96.226.18]] ([[User talk:86.96.226.18|talk]]) 16:54, 20 February 2013 (UTC)as phase, the amount of vapour present is quantified by the [[partial pressure]] of the gas. Also, vapours obey the [[barometric formula]] in a gravitational field just as conventional atmospheric gases do. |
||
== Vapours of flammable liquids == |
== Vapours of flammable liquids == |
||
| Line 108: | Line 91: | ||
[[yi:פארע]] |
[[yi:פארע]] |
||
[[zh:蒸氣]] |
[[zh:蒸氣]] |
||
סباص{{Polytonic|<references />}} |
|||
Revision as of 16:54, 20 February 2013
etween gas and the critical point.|The vapour-liquid critical point in a pressure-temperature phase diagram is at the high-temperature extreme of the liquid-gas phase boundary. (The dotted green line gives the anomalous behaviour of water.)]]--86.96.226.18 (talk) 16:54, 20 February 2013 (UTC)

The vapour pressure is the equilibrium pressure from a liquid or a solid at a specific temperature. The equilibrium vapour pressure of a liquid or solid is not affected by the amount of contact with the liquid or solid interface.
The normal boiling point of a liquid is the temperature at which the vapour pressure is equal to one atmosphere (unit).[1]
For two-phase systems (e.g., two liquid phases), the vapour pressure of the system is the sum of the vapour pressures of the two liquids. In the absence of stronger inter-species attractions between like-like or like-unlike molecules, the vapour pressure follows Raoult's Law, which states that the partial pressure of each component is the product of the vapour pressure of the pure component and its mole fraction in the mixture. The total vapour pressure is the sum of the component partial pressures.[2]
Examples

- Perfumes contain chemicals that vapourise at different temperatures and at different rate in scent accords known as notes.
- Atmospheric water vapour is found near the earth's surface, and may condense into small liquid droplets and form meteorological phenomena such as fog, mist and haar.
- Mercury-vapour lamps and sodium vapour lamps produce light from atoms in excited states.
--86.96.226.18 (talk) 16:54, 20 February 2013 (UTC)
Measuring vapour
Since it is in the g--86.96.226.18 (talk) 16:54, 20 February 2013 (UTC)--86.96.226.18 (talk) 16:54, 20 February 2013 (UTC)--86.96.226.18 (talk) 16:54, 20 February 2013 (UTC)as phase, the amount of vapour present is quantified by the partial pressure of the gas. Also, vapours obey the barometric formula in a gravitational field just as conventional atmospheric gases do.
Vapours of flammable liquids
Flammable liquids do not burn when ignited. It is the vapour cloud above the liquid that will burn if the vapour's concentration is between the Lower Flammable Limit (LFL) and Upper Flammable Limit (UFL) of the flammable liquid.
See also
- Evapouration
- Water vapour
- Dilution (equation)
- Vapour pressure
- Vapour Trail
- Vapouriser
- Gas phase
- Henry's Law
- Raoult's Law
References
סباص