Vanadium pentafluoride
| |
| Names | |
|---|---|
| IUPAC name
Vanadium(V) fluoride
| |
| Other names
Vanadium pentafluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.029.112 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| VF5 | |
| Molar mass | 145.934 |
| Appearance | colorless solid |
| Density | 2.502 g/cm3 (solid) |
| Melting point | 19.5 °C (67.1 °F; 292.6 K) |
| Boiling point | 48.3 °C (118.9 °F; 321.4 K) |
| Related compounds | |
Other cations
|
Niobium(V) fluoride Tantalum(V) fluoride |
Related Vanadium compounds
|
Vanadium(V) oxide Vanadium trifluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Vanadium(V) fluoride is the inorganic compound with the chemical formula VF5. It is a colorless volatile liquid[1] that freezes near room temperature. It is a highly reactive compound, as indicated by its ability to fluorinate organic substances.[2]
Properties and structure
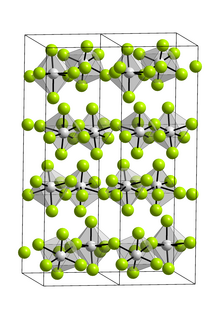
[edit]The compound is exclusively a monomer in the gas phase.[3] In the gas phase it adopts D3h symmetric trigonal bipyramidal geometry as indicated by electron diffraction.[4] As a solid, VF5 forms a polymeric structure with fluoride-bridged octahedral vanadium centers.[3][5]
The formation enthalpy of VF5 is -1429.4 ± 0.8 kJ/mol.[6]
It is the only known pentahalide of vanadium.
Synthesis
[edit]Vanadium pentafluoride can be prepared by fluorination of vanadium metal:[7][1]
- 2 V + 5 F2 → 2 VF5
Alternatively, disproportionation of vanadium tetrafluoride yields equal amounts of the solid trifluoride and the volatile pentafluoride:[8][9][1]
- 2 VF4 → VF3 + VF5
This conversion is conducted at 650 °C. It can also be synthesized by using elemental fluorine to fluorinate industrial concentrates and raw materials so as to produce VF5 on an industrial scale. VF5 can be synthesized from the reaction of raw materials such as metallic Vanadium, ferrovanadium, vanadium (V) oxide and vanadium tetrafluoride with elemental fluorine.[10]
VF5 ionises in the liquid state as reflected by the high values of Trouton's constant and electrical conductivities.[11]
Characteristics and reactivity
[edit]Interest in this highly corrosive compound began in the fifties when there were extensive studies of its physicochemical properties.[10] It is a powerful fluorinating and oxidizing agent. It oxidizes elemental sulfur to sulfur tetrafluoride:.
- S + 4 VF5 → 4 VF4 + SF4
Like other electrophilic metal halides, it hydrolyzes, first to the oxyhalide:
- VF5 + H2O → VOF3 + 2 HF
Then to the binary oxide:
- 2 VOF3 + 3 H2O → V2O5 + 6 HF
Hydrolysis is accelerated in the presence of base. Despite its tendency to hydrolyze, it can be dissolved in alcohols.
It is a Lewis acid, as illustrated by its formation of the hexafluorovanadate:[12][13][14]
- VF5 + KF → KVF6
Vanadium pentafluoride is a weaker acid and mainly undergoes oxidative and fluorinating reactions.[15]
The compound fluorinates unsaturated polyfluoroolefins into polyfluoroalkanes.[10]
The compound dissolves without reaction in liquid Cl2 and Br2. VF5 is moderately soluble in HF.
References
[edit]- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 989. ISBN 978-0-08-037941-8.
- ^ Canterford, J. H.; O'Donnell, Thomas A. (1967-03-01). "Reactivity of transition metal fluorides. IV. Oxidation-reduction reactions of vanadium pentafluoride". Inorganic Chemistry. 6 (3): 541–544. doi:10.1021/ic50049a025. ISSN 0020-1669.
- ^ a b Brownstein, S.; Latremouille, G. (1974-06-15). "Complex Fluoroanions in Solution. V. Vanadium Pentafluoride". Canadian Journal of Chemistry. 52 (12): 2236–2241. doi:10.1139/v74-323. ISSN 0008-4042.
- ^ Hagen, Kolbjoern.; Gilbert, Michael M.; Hedberg, Lise.; Hedberg, Kenneth. (1982-07-01). "Molecular structure of gaseous vanadium pentafluoride, VF5". Inorganic Chemistry. 21 (7): 2690–2693. doi:10.1021/ic00137a031. ISSN 0020-1669.
- ^ Brownstein, S. (1980-06-01). "The structure of VF5 in solution". Journal of Fluorine Chemistry. 15 (6): 539–540. doi:10.1016/S0022-1139(00)85231-8.
- ^ Nikitin, M. I.; Zbezhneva, S. G. (2014-12-16). "Thermochemistry of vanadium fluorides: The formation enthalpies of vanadium fluorides". High Temperature. 52 (6): 809–813. doi:10.1134/S0018151X14060108. ISSN 0018-151X. S2CID 98343689.
- ^ Trevorrow, L. E.; Fischer, J.; Steunenberg, R. K. (1957). "The Preparation and Properties of Vanadium Pentafluoride". Journal of the American Chemical Society. 79 (19): 5167–5168. doi:10.1021/ja01576a023.
- ^ Ruff, Otto; Lickfett, Herbert (1911). "Vanadinfluoride". Berichte der Deutschen Chemischen Gesellschaft. 44 (3): 2539–2549. doi:10.1002/cber.19110440379.
- ^ Cavell, R. G.; Clark, H. C. (1963). "Thermochemistry of vanadium fluorides". Transactions of the Faraday Society. 59: 2706. doi:10.1039/TF9635902706.
- ^ a b c Krasil'nikov, V. A.; Andreev, G. G.; Karelin, A. I.; Guzeeva, T. I.; Furin, G. G.; Bardin, V. V.; Avramenko, A. A. (1995-10-17). "ChemInform Abstract: Synthesis and Use of Vanadium Pentafluoride". ChemInform. 26 (42): no. doi:10.1002/chin.199542022. ISSN 1522-2667.
- ^ Clark, H. C.; Emeléus, H. J. (January 1958). "40. Chemical reactions with vanadium, niobium, and tantalum pentafluorides". J. Chem. Soc.: 190–195. doi:10.1039/jr9580000190.
- ^ Nikolsky, B. P. [Никольский, Б.П.] et al, eds. (1971). Справочник химика [The Chemist's Handbook] (in Russian). 3rd (corrected) ed. Leningrad: Khimiya.
- ^ Knunyants, I. L. [Кнунянц, И.Л.] et al, eds. (1995). Химическая энциклопедия [A Chemical Encyclopedia] (in Russian). Moscow: Soviet Encyclopedias. ISBN 978-5-85270-092-6
- ^ Lidin, R. A. [Лидин Р.А.] et al (2000). Химические свойства неорганических веществ: Учеб. пособие для вузов [Chemical Properties of Inorganic Substances: A University Textbook] (in Russian). 3rd (corrected) ed. Мoscow: Khimiya. ISBN 978-5-7245-1163-6
- ^ Fowler, Brian R.; Moss, Kenneth C. (1979-12-01). "An N.M.R. study of the solution chemistry of vanadium pentafluoride". Journal of Fluorine Chemistry. 14 (6): 485–494. doi:10.1016/S0022-1139(00)82524-5.
Other reading
[edit]- Arnold F. Holleman, Nils Wiberg: Lehrbuch der Anorganischen Chemie, 102. Auflage, de Gruyter, Berlin 2007, S. 1545, ISBN 978-3-11-017770-1.
