Ritter reaction
| Ritter reaction | |
|---|---|
| Named after | John J. Ritter |
| Reaction type | Addition reaction |
| Identifiers | |
| Organic Chemistry Portal | ritter-reaction |
| RSC ontology ID | RXNO:0000058 |
The Ritter reaction is a chemical reaction that transforms a nitrile into an N-alkyl amide using various electrophilic alkylating reagents. The original reaction formed the alkylating agent using an alkene in the presence of a strong acid.[1][2][3][4]
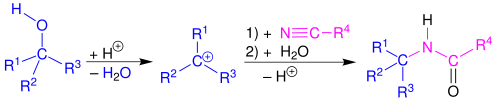
Mechanism and scope
[edit]The Ritter reaction proceeds by the electrophilic addition of either a carbenium ion or covalent species[5][6] to the nitrile. The resulting nitrilium ion is hydrolyzed to the desired amide.

Primary,[7] secondary,[4] tertiary,[8] and benzylic[9] alcohols,[1] as well as tert-butyl acetate,[10] also successfully react with nitriles in the presence of strong acids to form amides via the Ritter reaction. A wide range of nitriles can be used. In particular, formonitrile (hydrogen cyanide) can be used to prepare formamides, which are useful precursors to isocyanides.
Applications
[edit]The large scale application of the Ritter reaction is in the synthesis of tert-octylamine. An estimated 10,000 tons/y (year: 2000) of this and related lipophilic amines are prepared in this way.[11] Otherwise, the Ritter reaction is most useful in the formation of amines and amides of pharmaceutical interest. Real world applications include Merck's industrial-scale synthesis of anti-HIV drug Crixivan (indinavir);[12] the production of the falcipain-2 inhibitor PK-11195; the synthesis of the alkaloid aristotelone;[13] and synthesis of Amantadine, an antiviral and antiparkinsonian drug.[14] Other applications of the Ritter reaction include synthesis of dopamine receptor ligands[13] and production of racemic amphetamine from allylbenzene and methyl cyanide.[1][15]
The Ritter reaction is inferior to most amination methods because it cogenerates substantial amounts of salts. Illustrative is the conversion of isobutylene to tert-butylamine using HCN and sulfuric acid followed by base neutralization. The weight of the salt byproduct is greater than the weight of the amine.[11]
In the laboratory, the Ritter reaction suffers from the necessity of an extremely strong acid catalyst. Other methods have been proposed in order to promote carbocation formation, including photocatalytic electron transfer[16] or direct photolysis.[17]
History
[edit]The reaction is named after John J. Ritter, who supervised the Ph.D. thesis work of P. Paul Minieri.
- Ritter, John J.; Minieri, P. Paul (1948). "A New Reaction of Nitriles. I. Amides from Alkenes and Mononitriles". Journal of the American Chemical Society. 70 (12): 4045–8. doi:10.1021/ja01192a022. PMID 18105932.
- Ritter, John J.; Kalish, Joseph (1948). "A New Reaction of Nitriles. II. Synthesis of t-Carbinamines". Journal of the American Chemical Society. 70 (12): 4048–50. doi:10.1021/ja01192a023. PMID 18105933.
- Zil'berman, E. N. (1960). "Some reactions of nitriles with the formation of a new nitrogen–carbon bond". Russian Chemical Reviews. 29 (6): 331–340. Bibcode:1960RuCRv..29..331Z. doi:10.1070/RC1960v029n06ABEH001235. S2CID 250896801.
References
[edit]- ^ a b c Krimen, L.I.; Cota, Donald J. (1969). Adams, Rodger (ed.). Organic Reaction Volume 17. London: John Wiley & Sons, Inc. pp. 213–326. doi:10.1002/0471264180.or017.03. ISBN 9780471196150.
- ^ Johnson, Francis; Madroñero, Ramón (1966). "Heterocyclic Syntheses Involving Nitrilium Salts and Nitriles under Acidic Conditions". Advances in Heterocyclic Chemistry Volume 6. Vol. 6. pp. 95–146. doi:10.1016/S0065-2725(08)60576-0. ISBN 9780120206063.
- ^ Rappoport, Zvi; Meyers, A. I.; Sircar, J. C. (1970). The Cyano Group (1st ed.). Charlottesville, VA: Wiley Interscience. pp. 341–421. doi:10.1002/9780470771242.ch8. ISBN 9780471709138.
- ^ a b Bishop, Roger (1991). "Section 1.9 – Ritter-type Reactions". Comprehensive Organic Synthesis Volume 6: Heteroatom Manipulation. pp. 261–300. doi:10.1016/B978-0-08-052349-1.00159-1. ISBN 9780080359298.
{{cite book}}:|journal=ignored (help) - ^ Booth, Brian L.; Jibodu, Kehinde O.; Proença, M. Fernanda J. R. P. (1983). "The chemistry of nitrilium salts. Part 2. The preparation of nitrilium trifluoromethanesulphonate salts and their reactions with some oxygen and sulphur nucleophiles". Journal of the Chemical Society, Perkin Transactions 1: 1067–1073. doi:10.1039/P19830001067.
- ^ García Martínez, A. (1989). "An improved modification of ritter reaction". Tetrahedron Letters. 30 (51): 581–582. doi:10.1016/S0040-4039(00)95260-2.
- ^ Lebedev, Mikhail Y.; Erman, Mark B. (2002). "Lower primary alkanols and their esters in a Ritter-type reaction with nitriles. An efficient method for obtaining N-primary-alkyl amides". Tetrahedron Letters. 43 (8): 1397–1399. doi:10.1016/S0040-4039(02)00057-6.
- ^ Ritter, J.J.; Kalish, J. (1964). "α,α-Dimethyl-β-phenethylamine". Organic Syntheses. 42: 16. doi:10.15227/orgsyn.042.0016.
- ^ Parris, C.L. (1962). "N-Benzylacrylamide". Organic Syntheses. 42: 16. doi:10.15227/orgsyn.042.0016.
- ^ Fernholz, H.; Schmidt, H. J. (1969). "Tert-Butyl Acetate as Alkylating Agent". Angewandte Chemie International Edition in English. 8 (7): 521. doi:10.1002/anie.196905211.
- ^ a b Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2000). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001. ISBN 3527306730.
- ^ Clayden, J.; Greeves, N.; Warren, S.; Wothers, P. Organic Chemistry; Oxford Press: New York, 2001.
- ^ a b Kurti, L.; Czako, B. (2005). Strategic Applications of Named in Organic Synthesis. Burlington, MA Elsevier Academic Press.
- ^ Vardanyan, R.; Hruby, V.J. Synthesis of Essential Drugs, 1st Ed. Amsterdam: Elsevier, 2006; pp. 137
- ^ Fujisawa and Deguchi, Chemical Abstracts, 52, 11965 (1958)
- ^ Mattes, Susan L.; Farid, Samir (1980). "Photosensitized electron-transfer reactions of phenylacetylene". Journal of the Chemical Society, Chemical Communications (3): 126. doi:10.1039/C39800000126.
- ^ Kropp, Paul J.; Poindexter, Graham S.; Pienta, Norbert J.; Hamilton, David C. (1976). "Photochemistry of alkyl halides. 4. 1-Norbornyl, 1-norbornylmethyl, 1- and 2-adamantyl, and 1-octyl bromides and iodides". Journal of the American Chemical Society. 98 (25): 8135. doi:10.1021/ja00441a043.
