Saponin
Saponins (Latin "sapon", soap + "-in", one of) are bitter-tasting, usually toxic plant-derived secondary metabolites. They are organic chemicals and have a foamy quality when agitated in water and a high molecular weight. They are present in a wide range of plant species throughout the bark, leaves, stems, roots and flowers but particularly in soapwort (genus Saponaria), a flowering plant, the soapbark tree (Quillaja saponaria), common corn-cockle (Agrostemma githago L.), baby's breath (Gypsophila spp.) and soybeans (Glycine max L.). They are used in soaps, medicines (e.g. drug adjuvants), fire extinguishers, dietary supplements, steroid synthesis, and in carbonated beverages (for example, being responsible for maintaining the head on root beer). Saponins are both water and fat soluble, which gives them their useful soap properties. Some examples of these chemicals are glycyrrhizin (licorice flavoring) and quillaia (alt. quillaja), a bark extract used in beverages.[1][2][3]
Classification based on chemical structure
[edit]This section needs expansion. You can help by adding to it. (May 2023) |
Structurally, they are glycosides with at least one glycosidic linkage between a sugar chain (glycone) and another non-sugar organic molecule (aglycone).[citation needed]
Steroid glycosides
[edit]Steroid glycosides are saponins with 27-C atoms.[4] They are modified triterpenoids where their aglycone is a steroid, these compounds typically consist of a steroid aglycone attached to one or more sugar molecules, which can have various biological activities. These compounds are known for their significant cytotoxic, neurotrophic and antibacterial properties. These may also be used for partial synthesis of sex hormones or steroids.[5][1]
Triterpene glycosides
[edit]Triterpene glycosides, are natural glycosides present in various plants, herbs and sea cucumbers[6] and possess 30-C atoms.[4] These compounds consist of a triterpene aglycone attached to one or more sugar molecules. Triterpene glycosides exhibit a wide range of biological activities and pharmacological properties, making them valuable in traditional medicine and modern drug discovery.[1]
Uses
[edit]The saponins are a subclass of terpenoids, the largest class of plant extracts. The amphipathic nature of saponins gives them activity as surfactants with potential ability to interact with cell membrane components, such as cholesterol and phospholipids, possibly making saponins useful for development of cosmetics and drugs.[7] Saponins have also been used as adjuvants in development of vaccines,[8] such as Quil A, an extract from the bark of Quillaja saponaria.[7][9] This makes them of interest for possible use in subunit vaccines and vaccines directed against intracellular pathogens.[8] In their use as adjuvants for manufacturing vaccines, toxicity associated with sterol complexation remains a concern.[10]
Quillaja is toxic when consumed in large amounts, involving possible liver damage, gastric pain, diarrhea, or other adverse effects.[9] The NOAEL of saponins is around 300 mg/kg in rodents, so a dose of 3 mg/kg should be safe with a safety factor (see Therapeutic index) of 100.[11]
Saponins are used for their effects on ammonia emissions in animal feeding.[12] In the United States, researchers are exploring the use of saponins derived from plants to control invasive worm species, including the jumping worm.[13][14]
Decoction
[edit]The principal historical use of these plants was boiling down to make soap. Saponaria officinalis is most suited for this procedure, but other related species also work. The greatest concentration of saponin occurs during flowering, with the most saponin found in the woody stems and roots, but the leaves also contain some.
Biological sources
[edit]Saponins have historically been plant-derived, but they have also been isolated from marine organisms such as sea cucumber.[15] They derive their name from the soapwort plant (genus Saponaria, family Caryophyllaceae), the root of which was used historically as a soap.[1][16][2] In other representatives of this family, e.g. Agerostemma githago, Gypsophila spp., and Dianthus sp., saponins are also present in large quantities.[17] Saponins are also found in the botanical family Sapindaceae, including its defining genus Sapindus (soapberry or soapnut) and the horse chestnut, and in the closely related families Aceraceae (maples) and Hippocastanaceae. It is also found heavily in Gynostemma pentaphyllum (Cucurbitaceae) in a form called gypenosides, and ginseng or red ginseng (Panax, Araliaceae) in a form called ginsenosides. Saponins are also found in the unripe fruit of Manilkara zapota (also known as sapodillas), resulting in highly astringent properties. Nerium oleander (Apocynaceae), also known as White Oleander, is a source of the potent cardiac toxin oleandrin. Within these families, this class of chemical compounds is found in various parts of the plant: leaves, stems, roots, bulbs, blossom and fruit.[18] Commercial formulations of plant-derived saponins, e.g., from the soap bark tree, Quillaja saponaria, and those from other sources are available via controlled manufacturing processes, which make them of use as chemical and biomedical reagents.[19] Soyasaponins are a group of structurally complex oleanane-type triterpenoid saponins that include soyasapogenol (aglycone) and oligosaccharide moieties biosynthesized on soybean tissues. Soyasaponins were previously associated to plant-microbe interactions[20] from root exudates and abiotic stresses, as nutritional deficiency.[21]
Role in plant ecology and impact on animal foraging
[edit]In plants, saponins may serve as anti-feedants,[2] and to protect the plant against microbes and fungi.[citation needed] Some plant saponins (e.g., from oat and spinach) may enhance nutrient absorption and aid in animal digestion. However, saponins are often bitter to taste, and so can reduce plant palatability (e.g., in livestock feeds), or even imbue them with life-threatening animal toxicity. Some saponins are toxic to cold-blooded organisms and insects at particular concentrations. Further research is needed to define the roles of these natural products in their host organisms, which have been described as "poorly understood" to date.[22]
Ethnobotany
[edit]Most saponins, which readily dissolve in water, are poisonous to fish.[23] Therefore, in ethnobotany, they are known for their use by indigenous people in obtaining aquatic food sources. Since prehistoric times, cultures throughout the world have used fish-killing plants, typically containing saponins, for fishing.[24][25][26]
Although prohibited by law, fish-poison plants are still widely used by indigenous tribes in Guyana.[27]
On the Indian subcontinent, the Gondi people use poison-plant extracts in fishing.[28]
In 16th century, saponins-rich plant, Agrostemma githago, was used to treat ulcers, fistulas, and hemorrhages.[29]
Many of California's Native American tribes traditionally used soaproot (genus Chlorogalum), and/or the root of various yucca species, which contain saponin, as a fish poison. They would pulverize the roots, mix with water to generate a foam, then put the suds into a stream. This would kill or incapacitate the fish, which could be gathered easily from the surface of the water. Among the tribes using this technique were the Lassik, the Luiseño, and the Mattole.[30]
Chemical structure
[edit]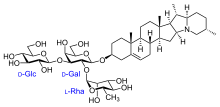
The vast heterogeneity of structures underlying this class of compounds makes generalizations difficult; they're a subclass of terpenoids, oxygenated derivatives of terpene hydrocarbons. Terpenes in turn are formally made up of five-carbon isoprene units (The alternate steroid base is a terpene missing a few carbon atoms). Derivatives are formed by substituting other groups for some of the hydrogen atoms of the base structure. In the case of most saponins, one of these substituents is a sugar, so the compound is a glycoside of the base molecule.[1]
More specifically, the lipophilic base structure of a saponin can be a triterpene, a steroid (such as spirostanol or furostanol) or a steroidal alkaloid (in which nitrogen atoms replace one or more carbon atoms). Alternatively, the base structure may be an acyclic carbon chain rather than the ring structure typical of steroids. One or two (rarely three) hydrophilic monosaccharide (simple sugar) units bind to the base structure via their hydroxyl (OH) groups. In some cases other substituents are present, such as carbon chains bearing hydroxyl or carboxyl groups. Such chain structures may be 1-11 carbon atoms long, but are usually 2–5 carbons long; the carbon chains themselves may be branched or unbranched.[1]
The most commonly encountered sugars are monosaccharides like glucose and galactose, though a wide variety of sugars occurs naturally. Other kinds of molecules such as organic acids may also attach to the base, by forming esters via their carboxyl (COOH) groups. Of particular note among these are sugar acids such as glucuronic acid and galacturonic acid, which are oxidized forms of glucose and galactose.[1]
See also
[edit]References
[edit]- ^ a b c d e f g Hostettmann K, Marston A (1995). Saponins. Cambridge: Cambridge University Press. p. 3ff. ISBN 978-0-521-32970-5. OCLC 29670810.
- ^ a b c "Cornell University Department of Animal Science". Cornell University. 14 August 2008. Archived from the original on 23 August 2015. Retrieved 23 February 2009.
- ^ Smakosz A, Matkowski A, Nawrot-Hadzik I (June 2024). "Phytochemistry and Biological Activities of Agrostemma Genus-A Review". Plants. 13 (12): 1673. doi:10.3390/plants13121673. PMC 11207627. PMID 38931105.
- ^ a b Vincken JP, Heng L, de Groot A, Gruppen H (1 February 2007). "Saponins, classification and occurrence in the plant kingdom". Phytochemistry. 68 (3): 275–297. doi:10.1016/j.phytochem.2006.10.008. ISSN 0031-9422.
- ^ Rao AV, Gurfinkel DM (2000). "The bioactivity of saponins: triterpenoid and steroidal glycosides". Drug Metabolism and Drug Interactions. 17 (1–4): 211–235. doi:10.1515/dmdi.2000.17.1-4.211. PMID 11201296.
- ^ Kim SK, Himaya SW (January 2012). Kim SK (ed.). "Triterpene glycosides from sea cucumbers and their biological activities". Advances in Food and Nutrition Research. Marine Medicinal Foods. 65. Academic Press: 297–319. doi:10.1016/b978-0-12-416003-3.00020-2. ISBN 978-0-12-416003-3. PMID 22361196.
- ^ a b Lorent JH, Quetin-Leclercq J, Mingeot-Leclercq MP (November 2014). "The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells". Organic & Biomolecular Chemistry. 12 (44). Royal Society of Chemistry: 8803–8822. doi:10.1039/c4ob01652a. PMID 25295776. S2CID 205925983.
- ^ a b Sun HX, Xie Y, Ye YP (March 2009). "Advances in saponin-based adjuvants". Vaccine. 27 (12): 1787–1796. doi:10.1016/j.vaccine.2009.01.091. PMID 19208455.
- ^ a b "Quillaja". Drugs.com. 2018. Archived from the original on 26 December 2018. Retrieved 26 December 2018.
- ^ Skene CD, Sutton P (September 2006). "Saponin-adjuvanted particulate vaccines for clinical use". Methods. 40 (1): 53–59. doi:10.1016/j.ymeth.2006.05.019. PMID 16997713.
- ^ Younes M, Aquilina G, Castle L, Engel KH, Fowler P, Frutos Fernandez MJ, et al. (March 2019). "Re-evaluation of Quillaia extract (E 999) as a food additive and safety of the proposed extension of use". EFSA Journal. 17 (3): e05622. doi:10.2903/j.efsa.2019.5622. PMC 7009130. PMID 32626248.
- ^ Zentner E (July 2011). "Effects of phytogenic feed additives containing quillaja saponaria on ammonia in fattening pigs" (PDF). Archived (PDF) from the original on 27 September 2013. Retrieved 27 November 2012.
- ^ Roach M (22 July 2020). "As Summer Takes Hold, So Do the Jumping Worms". The New York Times. ISSN 0362-4331. Archived from the original on 27 July 2020. Retrieved 30 July 2020.
- ^ "Invasive 'Jumping' Worms Are Now Tearing Through Midwestern Forests". Audubon. 2 January 2020. Archived from the original on 9 August 2020. Retrieved 30 July 2020.
- ^ Riguera R (August 1997). "Isolating bioactive compounds from marine organisms". Journal of Marine Biotechnology. 5 (4): 187–193.[dead link]
- ^ Birk Y, Peri I (1980). "Saponins". In Liener IE (ed.). Toxic constituents of plant foodstuffs (2nd ed.). New York City: Academic Press. p. 161. ISBN 978-0124499607.
- ^ Smakosz A, Matkowski A, Nawrot-Hadzik I (June 2024). "Phytochemistry and Biological Activities of Agrostemma Genus-A Review". Plants. 13 (12): 1673. doi:10.3390/plants13121673. PMC 11207627. PMID 38931105.
- ^ "Species Information". Dr. Duke's Phytochemical and Ethnobotanical Databases. Archived from the original on 18 February 2013. Retrieved 22 January 2015.
- ^ "Saponin from quillaja bark". Sigma-Aldrich. Archived from the original on 17 March 2022. Retrieved 23 February 2022.
- ^ Tsuno Y, Fujimatsu T, Endo K, Sugiyama A, Yazaki K (February 2018). "Soyasaponins: A New Class of Root Exudates in Soybean (Glycine max)". Plant & Cell Physiology. 59 (2): 366–375. doi:10.1093/pcp/pcx192. PMID 29216402.
- ^ Cotrim GD, Silva DM, Graça JP, Oliveira Junior A, Castro C, Zocolo GJ, et al. (January 2023). "Glycine max (L.) Merr. (Soybean) metabolome responses to potassium availability". Phytochemistry. 205: 113472. Bibcode:2023PChem.205k3472C. doi:10.1016/j.phytochem.2022.113472. PMID 36270412. S2CID 253027906.
- ^ Foerster H (22 May 2006). "MetaCyc Pathway: saponin biosynthesis I". Archived from the original on 15 September 2019. Retrieved 23 February 2009.
- ^ Howes FN (1930), "Fish-poison plants", Bulletin of Miscellaneous Information (Royal Gardens, Kew), 1930 (4): 129–153, doi:10.2307/4107559, JSTOR 4107559
- ^ Cannon JG, Burton RA, Wood SG, Owen NL (2004), "Naturally Occurring Fish Poisons from Plants", J. Chem. Educ., 81 (10): 1457, Bibcode:2004JChEd..81.1457C, doi:10.1021/ed081p1457
- ^ Bradley CE (1956), "Arrow and fish poison of the American southwest", Division of Biology, California Institute of Technology, vol. 10, no. 4, pp. 362–366, doi:10.1007/BF02859766, S2CID 35055877
- ^ Webb LJ, Tracey JG, Haydock KP (1959), An Australian phytochemical survey. III. Saponins in eastern Australian flowering plants, CSIRO, p. 26, doi:10.25919/5xj5-7648
- ^ Van Andel T (2000). "The diverse uses of fish-poison plants in Northwest Guyana". Economic Botany. 54 (4): 500–512. Bibcode:2000EcBot..54..500V. doi:10.1007/BF02866548. hdl:1874/23514. S2CID 24945604.
- ^ Murthy EN, Pattanaik C, Reddy CS, Raju VS (March 2010), "Piscicidal plants used by Gond tribe of Kawal wildlife sanctuary, Andhra Pradesh, India", Indian Journal of Natural Products and Resources, 1 (1): 97–101, archived from the original on 21 July 2011, retrieved 22 September 2010
- ^ Smakosz A, Matkowski A, Nawrot-Hadzik I (June 2024). "Phytochemistry and Biological Activities of Agrostemma Genus-A Review". Plants. 13 (12): 1673. doi:10.3390/plants13121673. PMC 11207627. PMID 38931105.
- ^ Campbell P (1999). Survival skills of native California. Gibbs Smith. p. 433. ISBN 978-0-87905-921-7. Archived from the original on 28 February 2022. Retrieved 20 November 2020.
