Tonicity: Difference between revisions
ClueBot NG (talk | contribs) m Reverting possible vandalism by 199.182.67.66 to version by 91.125.85.51. False positive? Report it. Thanks, ClueBot NG. (1263832) (Bot) |
|||
| Line 12: | Line 12: | ||
===Hypotonic=== |
===Hypotonic=== |
||
A hypotonic solution is a solution with a lower concentration of solutes. A cell in a hypotonic solution has a higher concentration of solutes than the surrounding solution, |
A hypotonic solution is a solution with a lower concentration of solutes. A cell in a hypotonic solution has a higher concentration of solutes than the surrounding solution, 169236484482374638264439298634causing water to flow into the cell. Too much inflow of water will lead the cell to lyse or to a phenomenon known as hemolysis. |
||
===Hypertonic=== |
===Hypertonic=== |
||
Revision as of 18:00, 10 October 2012
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages)
|
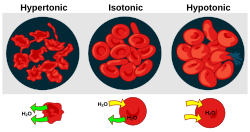

Tonicity is a measure of the osmotic pressure gradient (as defined by the water potential of the two solutions) of two solutions separated by a semipermeable membrane. It is commonly used when describing the response of cells immersed in an external solution. Like osmotic pressure, tonicity is influenced only by solutes that cannot cross the membrane, as only these exert an osmotic pressure. Solutes able to freely cross the membrane do not affect tonicity because they will always be in equal concentrations on both sides of the membrane.
There are three classifications of tonicity that one solution can have relative to another. The three are hypertonic, hypotonic, and isotonic.[1]
Hypotonic
A hypotonic solution is a solution with a lower concentration of solutes. A cell in a hypotonic solution has a higher concentration of solutes than the surrounding solution, 169236484482374638264439298634causing water to flow into the cell. Too much inflow of water will lead the cell to lyse or to a phenomenon known as hemolysis.
Hypertonic
Hypertonic refers to a higher concentration or has a greater degree of tone or tension. A cell in a hypertonic solution has a lower concentration of solutes than its surroundings, leading to a decrease of water in the cell. Water will leave the cell by means of osmosis and cause the cell to crenate. Crenation is a crinkling up process.
Plant cells may experience plasmolysis when placed in a hypertonic solution. Plasmolysis is the term which describes plant cells when the cytoplasm shrinks from the cell wall in a hypertonic environment. In plasmolysis, the cell wall stays intact, but the plasma membrane shrinks and the chloroplasts of the plant cell concentrate in the center of the cell.
Clarification
Especially when learning these definitions for the first time, it can be really hard to remember which is which, hyper and hypotonic. The picture to the right shows the effects of different solutions on cells. Note that if a cell is in a solution that is hypertonic, the cell itself is hypotonic and becomes wrinkled. Keeping track of which solution you're referencing (inside or outside the cell) is key.
Isotonic
An isotonic solution is a solution in which its effective osmole concentration is the same as the solute concentration of another solution with which it is compared. This occurs, for example, when the concentration of both water and total solute molecules are the same in an external solution as in the cell content. Water molecules diffuse through the plasma membrane in both directions, and as the rate of water diffusion is the same in both direction that cell will neither gain nor lose water.
See also
Notes
- ^ Sperelakis, Nicholas (2011). Cell Physiology Source Book: Essentials of Membrane Biophysics. Academic Press. p. 288. ISBN 978-0-12-387738-3.
