Tinzaparin sodium
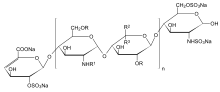 n = 1 to 25, R = H or SO3Na, R1 = H, SO3Na or COCH3, R2 = H and R3 = COONa or R2 = COONa and R3 = H | |
| Clinical data | |
|---|---|
| Trade names | innohep(R) |
| AHFS/Drugs.com | Monograph |
| Routes of administration | subcutaneous (once daily) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90% for Anti-Xa activity, 67% for Anti-IIa activity)[2] |
| Metabolism | minor metabolisation in liver by desulfation and/or depolymerization; excretion via kidneys in almost unchanged form |
| Elimination half-life | 200 min. for Anti-Xa activity, 257. min for Anti-IIa activity [3] |
| Identifiers | |
| CAS Number |
|
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.110.590 |
| Chemical and physical data | |
| Molar mass | 6500 g/mol (average)[4] |
| | |
Tinzaparin is an antithrombotic drug in the heparin group. It is a low molecular weight heparin (LMWH) marketed as Innohep worldwide. It has been approved by the U.S. Food and Drug Administration (FDA) for once daily treatment and prophylaxis of deep vein thrombosis (DVT) and pulmonary embolism (PE).[5]
It can be given subcutaneously by syringe, or intravenously.[6] It was manufactured by Leo pharmaceutical company, who withdrew the product from the US in 2011 due to low sales and a contamination issue.[7]
Use in elderly
[edit]In July 2008, the company revised the prescribing information to restrict the use of tinzaparin in patients 90 years of age or older. FDA is concerned that the preliminary data from the IRIS study suggests that the increased risk of mortality is not limited only to patients 90 years of age or older.
According to the study Innohep increases the risk of death for elderly patients (i.e., 70 years of age and older) with chronic kidney disease. Healthcare professionals should consider the use of alternative treatments to Innohep when treating elderly patients over 70 years of age with chronic kidney disease and deep vein thrombosis, pulmonary embolism, or both.
Use in pregnancy
[edit]No LMWH, except tinzaparin, is licensed for use in gestational hypercoagulability.[8] Still, tinzaparin is often the LMWH of choice in pregnant women.[8]
Side effects
[edit]Bleeding in overdose. There is occasionally bruising at the site of injection.
Monitoring
[edit]Tinzaparin does not affect the international normalized ratio (INR), prothrombin time (PT).[citation needed] Anti-factor Xa levels can be measured, and are often used to monitor tinzaparin.
Reversal agent
[edit]Protamine sulfate will reverse tinzaparin by 85% per package insert.
References
[edit]- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Cheer S.M. et al. Drugs 2004; 64 (13): 1479–1502
- ^ Pedersen P.C. et al. Thromb Res 1991; 61 (5-6): 477-487
- ^ European Pharmacopoeia, 6th Edition, 2008
- ^ Hull et al. NEJM 1992;326,15:975-982
- ^ Farmaceutiska Specialiteter i Sverige - the Swedish official drug catalog. Fass.se Archived 21 January 2011 at the Wayback Machine > Innohep
- ^ "Drug Shortages List". Archived from the original on 5 October 2016. Retrieved 4 October 2016.
- ^ a b "Archived copy". Archived from the original on 12 June 2010. Retrieved 15 May 2010.
{{cite web}}: CS1 maint: archived copy as title (link) Therapeutic anticoagulation in pregnancy. Norfolk and Norwich University Hospital (NHS Trust). Reference number CA3017. 9 June 2006 [review June 2009]
- (22)ESHRE April-2011 volume 33 pages 12–13-14
- e-medicine 2011
- RCOG March-2010 (Royal college for Obestetric and Gynecology)
- DVT.org/cardiologist
- Hull, New England Journal of Medicine, 2010 volume 22 page 19
External links
[edit]- tinzaparin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Sprigg N, Gray LJ, Bath PM, et al. (2007). "Early recovery and functional outcome are related with causal stroke subtype: data from the tinzaparin in acute ischemic stroke trial". Journal of Stroke and Cerebrovascular Diseases. 16 (4): 180–4. doi:10.1016/j.jstrokecerebrovasdis.2007.02.003. PMID 17689415.
