Surfactant: Difference between revisions
Undid revision 406900272 by 96.27.22.48 (talk) rv, unexplained deletion of sourced material |
moving text to proper section; needs citation, as well |
||
| Line 11: | Line 11: | ||
[[Thermodynamics]] of the surfactant systems are of great importance, theoretically and practically.<ref>{{Cite journal| author= Baeurle SA, Kroener J | title= Modeling Effective Interactions of Micellar Aggregates of Ionic Surfactants with the Gauss-Core Potential | journal=J. Math. Chem. | year=2004 | volume=36 | pages=409–421 | doi=10.1023/B:JOMC.0000044526.22457.bb}}</ref> This is because surfactant systems represent systems between ordered and disordered states of matter. Surfactant solutions may contain an ordered phase ([[micelle]]s) and a disordered phase (free surfactant molecules and/or [[ion]]s in the solution). |
[[Thermodynamics]] of the surfactant systems are of great importance, theoretically and practically.<ref>{{Cite journal| author= Baeurle SA, Kroener J | title= Modeling Effective Interactions of Micellar Aggregates of Ionic Surfactants with the Gauss-Core Potential | journal=J. Math. Chem. | year=2004 | volume=36 | pages=409–421 | doi=10.1023/B:JOMC.0000044526.22457.bb}}</ref> This is because surfactant systems represent systems between ordered and disordered states of matter. Surfactant solutions may contain an ordered phase ([[micelle]]s) and a disordered phase (free surfactant molecules and/or [[ion]]s in the solution). |
||
| ⚫ | Ordinary |
||
The [[spectroscopy]] and [[analytical chemistry]] of individual surfactant compounds is given in multiple references, including a multi-volume analysis reference textbook <ref name="Workman JJ">{{Cite book|author=Workman JJ |title=The Academic Press Handbook of Organic Compounds: NIR, IR, Raman, and UV-VIS Spectra Featuring Polymers, and Surfactants (3 volumes) |edition=1st |year=2000 |location=Boston, Mass.|publisher=Academic Press/Elsevier}}{{Page needed|date=September 2010}}</ref> |
The [[spectroscopy]] and [[analytical chemistry]] of individual surfactant compounds is given in multiple references, including a multi-volume analysis reference textbook <ref name="Workman JJ">{{Cite book|author=Workman JJ |title=The Academic Press Handbook of Organic Compounds: NIR, IR, Raman, and UV-VIS Spectra Featuring Polymers, and Surfactants (3 volumes) |edition=1st |year=2000 |location=Boston, Mass.|publisher=Academic Press/Elsevier}}{{Page needed|date=September 2010}}</ref> |
||
| Line 151: | Line 149: | ||
The two major surfactants used in the year 2000 were linear alkylbenzene [[sulphonate]]s (LAS) and the alkyl phenol [[ethoxylate]]s (APE). They break down in the [[aerobic]] conditions found in [[sewage treatment]] plants and in soil.<ref name="Scott2000">{{cite journal|last=Scott|first=M|year=2000|title=The biodegradation of surfactants in the environment|journal=Biochimica et Biophysica Acta (BBA) - Biomembranes|volume=1508|issue=1-2|pages=235–251|issn=00052736|doi=10.1016/S0304-4157(00)00013-7|accessdate=24 December 2010}}</ref> |
The two major surfactants used in the year 2000 were linear alkylbenzene [[sulphonate]]s (LAS) and the alkyl phenol [[ethoxylate]]s (APE). They break down in the [[aerobic]] conditions found in [[sewage treatment]] plants and in soil.<ref name="Scott2000">{{cite journal|last=Scott|first=M|year=2000|title=The biodegradation of surfactants in the environment|journal=Biochimica et Biophysica Acta (BBA) - Biomembranes|volume=1508|issue=1-2|pages=235–251|issn=00052736|doi=10.1016/S0304-4157(00)00013-7|accessdate=24 December 2010}}</ref> |
||
| ⚫ | Ordinary dishwashing [[detergent]], for example, will promote water penetration in soil, but the effect would only last a few days (many standard laundry detergent powders contain levels of chemicals such as [[alkali]] and [[chelation|chelating agents]], which can be damaging to plants and should not be applied to soils). Commercial soil wetting agents will continue to work for a considerable period, but they will eventually be degraded by soil micro-organisms. Some can, however, interfere with the life-cycles of some aquatic organisms, so care should be taken to prevent run-off of these products into streams, and excess product should not be washed down.{{citation needed}} |
||
==See also== |
==See also== |
||
Revision as of 01:41, 19 January 2011
Surfactants are compounds that lower the surface tension of a liquid, allowing easier spreading, and lowering of the interfacial tension between two liquids, or between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, and dispersants.
Etymology
The term surfactant is a blend of surface active agent.[1] Surfactants are usually organic compounds that are amphiphilic, meaning they contain both hydrophobic groups (their tails) and hydrophilic groups (their heads). Therefore, a surfactant molecule contains both a water insoluble (or oil soluble component) and a water soluble component. Surfactant molecules will migrate to the water surface, where the insoluble hydrophobic group may extend out of the bulk water phase, either into the air or, if water is mixed with an oil, into the oil phase, while the water soluble head group remains in the water phase. This alignment and aggregation of surfactant molecules at the surface, acts to alter the surface properties of water at the water/air or water/oil interface.
In Index Medicus and the United States National Library of Medicine, surfactant is reserved for the meaning pulmonary surfactant. For the more general meaning, surface active agent is the heading.
Properties
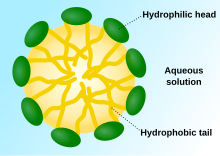
Surfactants reduce the surface tension of water by adsorbing at the liquid-gas interface. They also reduce the interfacial tension between oil and water by adsorbing at the liquid-liquid interface. Many surfactants can also assemble in the bulk solution into aggregates. Examples of such aggregates are vesicles and micelles. The concentration at which surfactants begin to form micelle is known as the critical micelle concentration (CMC). When micelles form in water, their tails form a core that can encapsulate an oil droplet, and their (ionic/polar) heads form an outer shell that maintains favorable contact with water. When surfactants assemble in oil, the aggregate is referred to as a reverse micelle. In a reverse micelle, the heads are in the core and the tails maintain favorable contact with oil. Surfactants are also often classified into four primary groups; anionic, cationic, non-ionic, and zwitterionic (dual charge).
Thermodynamics of the surfactant systems are of great importance, theoretically and practically.[2] This is because surfactant systems represent systems between ordered and disordered states of matter. Surfactant solutions may contain an ordered phase (micelles) and a disordered phase (free surfactant molecules and/or ions in the solution).
The spectroscopy and analytical chemistry of individual surfactant compounds is given in multiple references, including a multi-volume analysis reference textbook [3]
Applications and sources
Surfactants play an important role as cleaning, wetting, dispersing, emulsifying, foaming and anti-foaming agents in many practical applications and products, including:
- Detergents
- Fabric softeners
- Emulsions
- Paints
- Adhesives
- Inks
- Anti-fogs
- Ski waxes, snowboard wax
- Deinking of recycled papers, both in flotation, washing and enzymatic processes
- Laxatives
- Agrochemical formulations
- Herbicides some
- Insecticides
- Quantum dot coatings
- Biocides (sanitizers)
- Cosmetics:
- Shampoos
- Hair conditioners (after shampoo)
- Toothpastes
- Spermicides (nonoxynol-9)
- Firefighting
- Pipelines, liquid drag reducing agent
- Alkali Surfactant Polymers (used to mobilize oil in oil wells)
- Ferrofluids
- Leak Detectors
Detergents in biochemistry and biotechnology
In solution: detergents help solubilize molecules by dissociating aggregates and unfolding proteins. These include SDS, CTBA. Detergents are key reagents to extract protein by lysis of the cells and tissues: they disorganize the membranes lipidic bilayer (SDS, Triton X-100, X-114, CHAPS, DOC, NP-40), and solubilize proteins. Milder detergents such as (OctylThioGlucosides) are used to solubilize sensible proteins (enzymes, receptors). Non-solubilized material is harvested by centrifugation or other means. For electrophoresis i.e., proteins are classically treated with SDS to denature the native tertiary and quaternary structures, allowing the separation of proteins according to their molecular weight.
Detergents have also been used to decellularise organs. This process maintains a matrix of proteins that preserves the structure of the organ and often the microvascular network. The process has been successfully used to prepare organs such as the liver and heart for transplant in rats.[4] Pulmonary surfactants are also naturally secreted by type II cells of the lung alveoli in mammals.
Classification
According to the composition of their tail
The tail of surfactants can be:
- A hydrocarbon chain: aromatic hydrocarbons (arenes), alkanes (alkyl), alkenes, cycloalkanes, alkyne-based;
- An alkyl ether chain:
- Ethoxylated surfactants: polyethylene oxides are inserted to increase the hydrophilic character of a surfactant;
- Propoxylated surfactants: polypropylene oxides are inserted to increase the lipophilic character of a surfactant;
- A fluorocarbon chain: fluorosurfactants;
- A siloxane chain: siloxane surfactants
Surfactant can have one or two tails (double chained surfactants).
According to the composition of their head

A surfactant can be classified by the presence of formally charged groups in its head. A non-ionic surfactant has no charge groups in its head. The head of an ionic surfactant carries a net charge. If the charge is negative, the surfactant is more specifically called anionic; if the charge is positive, it is called cationic. If a surfactant contains a head with two oppositely charged groups, it is termed zwitterionic.
Some commonly encountered surfactants of each type include:
- Ionic
- Anionic: based on permanent anions (sulfate, sulfonate, phosphate) or pH-dependent anions (carboxylate):
- Sulfates:
- Alkyl sulfates: ammonium lauryl sulfate, sodium lauryl sulfate (SDS);
- Alkyl ether sulfates: sodium laureth sulfate, also known as sodium lauryl ether sulfate (SLES), sodium myreth sulfate;
- Sulfonates:
- Docusates: dioctyl sodium sulfosuccinate;
- Sulfonate fluorosurfactants: perfluorooctanesulfonate (PFOS), perfluorobutanesulfonate;
- Alkyl benzene sulfonates;
- Phosphates:
- Alkyl aryl ether phosphate
- Alkyl ether phosphate
- Carboxylates:
- Alkyl carboxylates: Fatty acid salts (soaps): sodium stearate;
- Sodium lauroyl sarcosinate;
- Carboxylate fluorosurfactants: perfluorononanoate, perfluorooctanoate (PFOA or PFO)
- Sulfates:
- Anionic: based on permanent anions (sulfate, sulfonate, phosphate) or pH-dependent anions (carboxylate):
- Cationic: based on:
- pH-dependent primary, secondary or tertiary amines: primary amines become positively charged at pH < 10, secondary amines become charged at pH < 4:
- Permanently charged quaternary ammonium cation:
- Alkyltrimethylammonium salts: cetyl trimethylammonium bromide (CTAB) a.k.a. hexadecyl trimethyl ammonium bromide, cetyl trimethylammonium chloride (CTAC);
- Cetylpyridinium chloride (CPC);
- Polyethoxylated tallow amine (POEA);
- Benzalkonium chloride (BAC);
- Benzethonium chloride (BZT);
- 5-Bromo-5-nitro-1,3-dioxane;
- Dimethyldioctadecylammonium chloride
- Dioctadecyldimethylammonium bromide (DODAB)
- Cationic: based on:
- Zwitterionic (amphoteric): based on primary, secondary or tertiary amines or quaternary ammonium cation with:
- Sulfonates:
- CHAPS (3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate);
- Sultaines: cocamidopropyl hydroxysultaine;
- Carboxylates:
- Phosphates: lecithin
- Sulfonates:
- Zwitterionic (amphoteric): based on primary, secondary or tertiary amines or quaternary ammonium cation with:
- Nonionic
- Fatty alcohols:
- Cetyl alcohol,
- Stearyl alcohol,
- Cetostearyl alcohol (consisting predominantly of cetyl and stearyl alcohols),
- Oleyl alcohol;
- Polyoxyethylene glycol alkyl ethers (Brij): CH3–(CH2)10–16–(O-C2H4)1–25–OH:
- Polyoxypropylene glycol alkyl ethers: CH3–(CH2)10–16–(O-C3H6)1–25–OH;
- Glucoside alkyl ethers: CH3–(CH2)10–16–(O-Glucoside)1–3–OH:
- Polyoxyethylene glycol octylphenol ethers: C8H17–(C6H4)–(O-C2H4)1–25–OH:
- Polyoxyethylene glycol alkylphenol ethers: C9H19–(C6H4)–(O-C2H4)1–25–OH:
- Glycerol alkyl esters:
- Polyoxyethylene glycol sorbitan alkyl esters: Polysorbates;
- Sorbitan alkyl esters: Spans;
- Cocamide MEA, cocamide DEA;
- Dodecyl dimethylamine oxide;
- Block copolymers of polyethylene glycol and polypropylene glycol: Poloxamers
- Fatty alcohols:
According to the composition of their counter-ion
In the case of ionic surfactants, the counter-ion can be:
- Monoatomic / Inorganic:
- Cations: metals : alkali metal, alkaline earth metal, transition metal;
- Anions: halides: chloride (Cl−), bromide (Br−), iodide (I−);
- Polyatomic / Organic:
- Cations: ammonium, pyridinium, triethanolamine (TEA)
- Anions: tosyls, trifluoromethanesulfonates, methylsulfate
Current market
The annual global production of surfactants was 13 million metric tons in 2008 and the annual turnover reached US$24.33 billion in 2009, nearly 2% up from the previous year. The market is expected to experience quite healthy growth by 2.8% annually to 2012 and by 3.5 - 4% thereafter.[5] [6]
Health and environmental controversy
Some surfactants are known to be toxic to animals, ecosystems and humans, and can increase the diffusion of other environmental contaminants.[7][8][9] Despite this, they are routinely deposited in numerous ways on land and into water systems, whether as part of an intended process or as industrial and household waste. Some surfactants have proposed or voluntary restrictions on their use. For example, PFOS is a persistent organic pollutant as judged by the Stockholm Convention. Additionally, PFOA has been subject to a voluntary agreement by the U.S. Environmental Protection Agency and eight chemical companies to reduce and eliminate emissions of the chemical and its precursors.[10]
The two major surfactants used in the year 2000 were linear alkylbenzene sulphonates (LAS) and the alkyl phenol ethoxylates (APE). They break down in the aerobic conditions found in sewage treatment plants and in soil.[11]
Ordinary dishwashing detergent, for example, will promote water penetration in soil, but the effect would only last a few days (many standard laundry detergent powders contain levels of chemicals such as alkali and chelating agents, which can be damaging to plants and should not be applied to soils). Commercial soil wetting agents will continue to work for a considerable period, but they will eventually be degraded by soil micro-organisms. Some can, however, interfere with the life-cycles of some aquatic organisms, so care should be taken to prevent run-off of these products into streams, and excess product should not be washed down.[citation needed]
See also
References
- ^ Rosen MJ (2004). Surfactants and Interfacial Phenomena (3rd ed.). Hoboken, New Jersey: John Wiley & Sons.[page needed]
- ^ Baeurle SA, Kroener J (2004). "Modeling Effective Interactions of Micellar Aggregates of Ionic Surfactants with the Gauss-Core Potential". J. Math. Chem. 36: 409–421. doi:10.1023/B:JOMC.0000044526.22457.bb.
- ^ Workman JJ (2000). The Academic Press Handbook of Organic Compounds: NIR, IR, Raman, and UV-VIS Spectra Featuring Polymers, and Surfactants (3 volumes) (1st ed.). Boston, Mass.: Academic Press/Elsevier.[page needed]
- ^ Wein, Harrison (28 June 2010). "Progress Toward an Artificial Liver Transplant - NIH Research Matters". National Institutes of Health (NIH).
- ^ "Market Report: World Surfactant Market". Acmite Market Intelligence.
{{cite web}}: External link in|publisher= - ^ Reznik GO, Vishwanath P, Pynn MA, Sitnik JM, Todd JJ, Wu J, Jiang Y, Keenan BG, Castle AB, Haskell RF, Smith TF, Somasundaran P, Jarrell KA. (2010). "Use of sustainable chemistry to produce an acyl amino acid surfactant". Appl Microbiol Biotechnol. 86 (5): 1387–97. doi:10.1007/s00253-009-2431-8. PMID 20094712.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Metcalfe TL, Dillon PJ, Metcalfe CD (2008). "Detecting the transport of toxic pesticides from golf courses into watersheds in the Precambrian Shield region of Ontario, Canada". Environmental Toxicology and Chemistry. 27 (4): 811–8. doi:10.1897/07-216.1. PMID 18333674.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Emmanuel E, Hanna K, Bazin C, Keck G, Clément B, Perrodin Y (2005). "Fate of glutaraldehyde in hospital wastewater and combined effects of glutaraldehyde and surfactants on aquatic organisms". Environment International. 31 (3): 399–406. doi:10.1016/j.envint.2004.08.011. PMID 15734192.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Murphy MG, Al-Khalidi M, Crocker JF, Lee SH, O'Regan P, Acott PD (2005). "Two formulations of the industrial surfactant, Toximul, differentially reduce mouse weight gain and hepatic glycogen in vivo during early development: effects of exposure to Influenza B Virus". Chemosphere. 59 (2): 235–46. doi:10.1016/j.chemosphere.2004.11.084. PMID 15722095.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ USEPA: "2010/15 PFOA Stewardship Program" Accessed October 26, 2008.
- ^ Scott, M (2000). "The biodegradation of surfactants in the environment". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1508 (1–2): 235–251. doi:10.1016/S0304-4157(00)00013-7. ISSN 0005-2736.
{{cite journal}}:|access-date=requires|url=(help)
