Steroid: Difference between revisions
| [pending revision] | [pending revision] |
ClueBot NG (talk | contribs) m Reverting possible vandalism by 199.212.252.135 to version by Flyer22. False positive? Report it. Thanks, ClueBot NG. (1756799) (Bot) |
|||
| Line 10: | Line 10: | ||
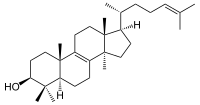
[[File:Lanosterin.svg|thumb|200px|[[Lanosterol]], the [[biosynthetic]] precursor to animal steroids. The total number of carbons (30) reflects its [[triterpenoid]] origin.]] |
[[File:Lanosterin.svg|thumb|200px|[[Lanosterol]], the [[biosynthetic]] precursor to animal steroids. The total number of carbons (30) reflects its [[triterpenoid]] origin.]] |
||
oh yeah |
|||
== Nomenclature == |
|||
Steroids are a class of [[organic compound]]s with a chemical structure that contains the core of [[gonane]] or a skeleton derived therefrom. Usually, [[methyl group]]s are present at the carbons C-10 and C-13 – an [[alkyl]] [[Side chain|side-chain]] at carbon C-17 may also be present. |
|||
[[Gonane]], see above, is the simplest possible steroid and is composed of seventeen [[carbon]] atoms, bonded together to form four fused rings. The three [[cyclohexane]] rings (designated as rings A, B, and C in the figure below) form the skeleton of [[phenanthrene]]; ring D has a [[cyclopentane]] structure. Hence, together they are called [[cyclopentaphenanthrene]].<ref>{{PubChem|130801}}; {{CAS|219-08-9|cyclopentaphenanthrene}}</ref> |
|||
{| |
|||
|- valign="top" |
|||
| [[File:Cholsäure.svg|thumb|x120px|The more complex structure of [[cholic acid]], a [[bile acid]].]] |
|||
|} |
|||
Commonly, steroids have a methyl group at the carbons C-10 and C-13 and an alkyl side chain at carbon C-17. Further, they vary by the configuration of the side chain, the number of additional methyl groups, and the functional groups attached to the rings. For example, [[sterol]]s have a [[hydroxyl group]] attached at position C-3. |
|||
The following are some examples of steroid structures: |
|||
{| |
|||
|- valign="top" |
|||
| [[File:Testosteron.svg|thumb|x100px|The [[anabolic steroid]] [[testosterone]], the principal male [[Sex steroid|sex hormone]].]] |
|||
| [[File:Progesteron.svg|thumb|x120px|[[Progesterone]], a steroid hormone involved in the female menstrual cycle, pregnancy and embryogenesis. ]] |
|||
| [[File:Medrogestone.png|thumb|x120px|[[Medrogestone]], a synthetic drug with similar effects as progesterone.]] |
|||
| [[File:Sitosterol structure.svg|thumb|x120px|[[beta-Sitosterol|β-Sitosterol]], a [[phytosterol]] showing the hydroxyl group at C-3. ]] |
|||
|} |
|||
== Types == |
== Types == |
||
Revision as of 14:17, 21 March 2014


A steroid is a type of organic compound that contains a characteristic arrangement of four cycloalkane rings that are joined to each other. Examples of steroids include the dietary lipid cholesterol, the sex hormones estradiol and testosterone and the anti-inflammatory drug dexamethasone.
The core of steroids is composed of seventeen carbon atoms bonded together that take the form of four fused rings: three cyclohexane rings (designated as rings A, B and C in the figure to the right) and one cyclopentane ring (the D ring). The steroids vary by the functional groups attached to this four-ring core and by the oxidation state of the rings. Sterols are special forms of steroids, with a hydroxyl group at position-3 and a skeleton derived from cholestane.[1]
Hundreds of distinct steroids are found in plants, animals and fungi. All steroids are made in cells either from the sterols lanosterol (animals and fungi, see below right) or from cycloartenol (plants). Both lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene.[2]
oh yeah
Types
The inclusion or exclusion of items from this list or length of this list is disputed. |
Taxonomical/functional
Some of the common categories of steroids:
- Animal
- Insect
- Ecdysteroids such as ecdysterone that controls moulting
- Vertebrate
- Steroid hormones
- Sex steroids are a subset of sex hormones that produce sex differences or support reproduction. They include androgens, estrogens, and progestagens.
- Corticosteroids include glucocorticoids and mineralocorticoids. Glucocorticoids regulate many aspects of metabolism and immune function, whereas mineralocorticoids help maintain blood volume and control renal excretion of electrolytes. Most medical 'steroid' drugs are corticosteroids.
- Anabolic steroids are a class of steroids that interact with androgen receptors to increase muscle and bone synthesis. There are natural and synthetic anabolic steroids. In popular language, the word "steroids" usually refers to anabolic steroids.
- Cholesterol, which modulates the fluidity of cell membranes and is the principal constituent of the plaques implicated in atherosclerosis.
- Steroid hormones
- Insect
- Plant
- Phytosterols
- Brassinosteroids (includes several plant hormones)
- Steroidal alkaloids found notably in Solanaceae
- Fungus
Structural
It is also possible to classify steroids based upon their chemical composition. One example of how MeSH performs this classification is available at the Wikipedia MeSH catalog. Examples from this classification include:
| Class | Examples | Number of carbon atoms |
|---|---|---|
| Cholestanes | cholesterol | 27 |
| Cholanes | cholic acid | 24 |
| Pregnanes | progesterone | 21 |
| Androstanes | testosterone | 19 |
| Estranes | estradiol | 18 |
Gonane (or steroid nucleus) is the parent (17-carbon tetracyclic) hydrocarbon molecule without any alkyl sidechains.[3]
Biological significance
Steroid and their metabolites are frequently used signalling molecules. The most notable examples are the steroid hormones.
Steroids along with phospholipids function as components of cell membranes. Steroids such as cholesterol decrease membrane fluidity.[4]
Similar to lipids, steroids represent highly concentrated energy stores. However steroids are not typically used as sources of energy. In mammals, they are normally metabolized and excreted.
Pharmacological actions
A number of drugs target the mevalonate pathway:
- Statins (used for elevated cholesterol levels)
- Bisphosphonates (used in treatment of various bone-degenerative diseases)
Biosynthesis and metabolism
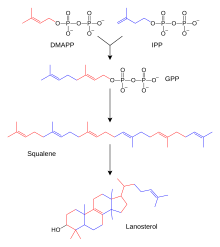
Steroid biosynthesis is an anabolic metabolic pathway that produces steroids from simple precursors. A unique biosynthetic pathway is followed in animals compared to many other organisms, making the pathway a common target for antibiotics and other anti-infective drugs. In addition, steroid metabolism in humans is the target of cholesterol-lowering drugs such as statins.
In humans and other animals, the biosynthesis of steroids follows the mevalonate pathway that uses acetyl-CoA as building-blocks to form dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP).[5] In subsequent steps, DMAPP and IPP are joined to form geranyl pyrophosphate (GPP), which in turn is used to synthesize the steroid lanosterol. Further modifications of lanosterol into other steroids are classified steroidogenesis transformations.
Mevalonate pathway
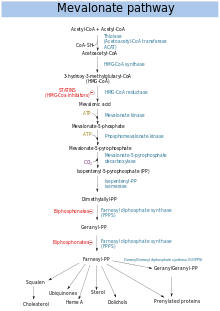
The mevalonate pathway or HMG-CoA reductase pathway starts with acetyl-CoA and ends with dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP).
DMAPP and IPP in turn donate isoprene units, which are assembled and modified to form terpenes and isoprenoids,[6] which are a large class of lipids that include the carotenoids, and form the largest class of plant natural products.[7]
Here, the isoprene units are joined together to make squalene and then folded up and formed into a set of rings to make lanosterol.[8] Lanosterol can then be converted into other steroids such as cholesterol and ergosterol.[8][9]
Steroidogenesis
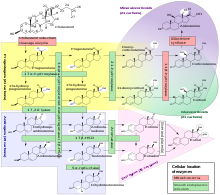
Steroidogenesis is the biological process by which steroids are generated from cholesterol and transformed into other steroids.[10] The pathways of steroidogenesis differ between different species – as an example the pathways of human steroidogenesis are shown in this figure below: Following is a list of the major classes of steroid hormones and some prominent members, with examples of major related functions:
- Progestogens:
- Progesterone, which regulates the cyclical changes of the endometrium of the uterus, and the maintenance of pregnancy
- Corticosteroids (Corticoids):
- Aldosterone (Mineralocorticoids), which contributes to the regulation of blood pressure
- Cortisol (Glucocorticoids), whose functions include acting as an immunosuppressant
- Androgens:
- Testosterone, which contributes to the development and maintenance of male secondary sex characteristics
- Estrogens:
- Estrogen, which contributes to the development and maintenance of female secondary sex characteristics
Locations of human steroidogenesis:
- Progestogens serve as precursors to all other human steroids – thus all human tissues which produce steroids must first convert cholesterol to pregnenolone. This conversion is the rate-limiting step of steroid synthesis, which occurs inside the mitochondrion of the respective tissue.[11]
- Corticosteroids are produced in the adrenal cortex.
- Estrogen and progesterone are made primarily in the ovary and in the placenta during pregnancy, and testosterone in the testes.
- Testosterone is also converted into estrogen to regulate the supply of each, in the bodies of both females and males.
- In addition, certain neurons and glia in the central nervous system (CNS) express the enzymes that are required for the local synthesis of pregnane neurosteroids, either de novo or from peripherally derived sources.
Regulation
Several key enzymes can be activated through DNA transcriptional regulation on activation of SREBP (Sterol Regulatory Element-Binding Protein-1 and -2).[citation needed] This intracellular sensor detects low cholesterol levels and stimulates endogenous production by the HMG-CoA reductase pathway, as well as increasing lipoprotein uptake by up-regulating the LDL receptor.[citation needed] Regulation of this pathway is also achieved by controlling the rate of translation of the mRNA, degradation of reductase and phosphorylation.[citation needed]
Alternative pathways
In plants and bacteria, the non-mevalonate pathway uses pyruvate and glyceraldehyde 3-phosphate as substrates.[6][12]
Metabolism
Steroids are oxidized mainly by cytochrome P450 oxidase enzymes, such as CYP3A4. These reactions introduce oxygen into the steroid ring and allow the structure to be broken up by other enzymes, to form bile acids as final products.[13] These bile acids can then be eliminated through secretion from the liver in the bile.[14] The expression of this oxidase gene can be upregulated by the steroid sensor PXR when there is a high blood concentration of steroids.[15]
Isolation and syntheses of steroids
Isolation of steroids
This section is empty. You can help by adding to it. (December 2013) |
Microbial transformations
This section needs expansion. You can help by adding to it. (March 2014) |
Phytosterols, for instance, mixtures of soybean sterols, can be used as starting materials and converted into two kinds of steroid hormone intermediates through microbial transformation.[citation needed] Microbial catabolism of phytosterol sidechains yields either C-19 steroids, a precursor to most steroid hormones including sex hormones, or C-22 steroids, a precursor to adrenocortical hormones.[16][17]
Partial and total chemical synthesis
This section needs expansion. You can help by adding to it. (March 2014) |
The chemical conversion of sapogenins to steroids—e.g., via the Marker degradation—is a method of partial synthesis that is a long-established alternative to microbial transformation of phytosterols to steroids, and underpinned Syntex efforts using the Mexican barbasco trade (harvesting and marketing large tubers of wild-growing plants, e.g., yams) to produce early synthetic steroids.[citation needed]
Determination of structure and methods of analysis
This section is empty. You can help by adding to it. (December 2013) |
History
A number of Nobel Prizes have been awarded for research involving steroids. These prizes include:
- 1927 (Chemistry) Heinrich Otto Wieland – constitution of the bile acids, sterols, and their connection with the vitamins[18]
- 1928 (Chemistry) Adolf Otto Reinhold Windaus – constitution of the sterols and their connection with the vitamins[19]
- 1939 (Chemistry) Adolf Butenandt and Leopold Ruzicka – isolation and structural studies of steroid sex hormones, and related studies on higher terpenes[20]
- 1950 (Physiology or Medicine) Edward Kendall, Tadeus Reichstein, Philip Hench – on the structure and biological effects of adrenal hormones[21]
- 1965 (Chemistry) Robert Burns Woodward, in part for the synthesis of cholesterol, cortisone, and lanosterol[22]
- 1969 (Chemistry) Derek Barton, Odd Hassel, development of the concept of conformation and its application in chemistry, where a specific important emphasis was on the conformation of the "Steroid Nucleus"[23]
- 1975 (Chemistry) Vladimir Prelog, in part for developing methods to determine the stereochemical course of cholesterol biosynthesis from mevalonic acid via squalene[24]
See also
- Batrachotoxin
- Corticosteroid
- List of steroid abbreviations
- Sex steroid
- Steroid hormone
- Steroid hydroxylases
- Steroid sulfatase
- Steroidogenic acute regulatory protein
References
- ^ a b c Moss GP (1989). "Nomenclature of Steroids (Recommendations 1989)". Pure & Appl. Chem. 61 (10): 1783–1822. doi:10.1351/pac198961101783. PDF "IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). The nomenclature of steroids. Recommendations 1989". Eur. J. Biochem. 186 (3): 429–58. December 1989. doi:10.1111/j.1432-1033.1989.tb15228.x. PMID 2606099.
- ^ "Lanosterol biosynthesis". Recommendations on Biochemical & Organic Nomenclature, Symbols & Terminology. International Union Of Biochemistry And Molecular Biology.
- ^ Edgren RA, Stanczyk FZ (December 1999). "Nomenclature of the gonane progestins". Contraception. 60 (6): 313. doi:10.1016/S0010-7824(99)00101-8. PMID 10715364.
- ^ Sadava D, Hillis DM, Heller HC, Berenbaum MR (2011). Life: The Science of Biology 9th Edition. San Francisco: Freeman. pp. 105–114. ISBN 1-4292-4646-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Grochowski L, Xu H, White R (2006). "Methanocaldococcus jannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate". J Bacteriol. 188 (9): 3192–8. doi:10.1128/JB.188.9.3192-3198.2006. PMC 1447442. PMID 16621811.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Kuzuyama T, Seto H (2003). "Diversity of the biosynthesis of the isoprene units". Nat Prod Rep. 20 (2): 171–83. doi:10.1039/b109860h. PMID 12735695.
- ^ Dubey V, Bhalla R, Luthra R (2003). "An overview of the non-mevalonate pathway for terpenoid biosynthesis in plants" (PDF). J Biosci. 28 (5): 637–46. doi:10.1007/BF02703339. PMID 14517367.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Schroepfer G (1981). "Sterol biosynthesis". Annu Rev Biochem. 50: 585–621. doi:10.1146/annurev.bi.50.070181.003101. PMID 7023367.
- ^ Lees N, Skaggs B, Kirsch D, Bard M (1995). "Cloning of the late genes in the ergosterol biosynthetic pathway of Saccharomyces cerevisiae—a review". Lipids. 30 (3): 221–6. doi:10.1007/BF02537824. PMID 7791529.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hanukoglu I (Dec 1992). "Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis". J Steroid Biochem Mol Biol. 43 (8): 779–804. doi:10.1016/0960-0760(92)90307-5. PMID 22217824.
- ^ Rossier MF (2006). "T channels and steroid biosynthesis: in search of a link with mitochondria". Cell Calcium. 40 (2): 155–64. doi:10.1016/j.ceca.2006.04.020. PMID 16759697.
- ^ Lichtenthaler H (1999). "The 1-Dideoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants". Annu Rev Plant Physiol Plant Mol Biol. 50: 47–65. doi:10.1146/annurev.arplant.50.1.47. PMID 15012203.
- ^ Pikuleva IA (2006). "Cytochrome P450s and cholesterol homeostasis". Pharmacol. Ther. 112 (3): 761–73. doi:10.1016/j.pharmthera.2006.05.014. PMID 16872679.
- ^ Zollner G, Marschall HU, Wagner M, Trauner M (2006). "Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations". Mol. Pharm. 3 (3): 231–51. doi:10.1021/mp060010s. PMID 16749856.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kliewer S, Goodwin B, Willson T (2002). "The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism". Endocr. Rev. 23 (5): 687–702. doi:10.1210/er.2001-0038. PMID 12372848.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Conner AH, Nagaoka M, Rowe JW, Perlman D (August 1976). "Microbial conversion of tall oil sterols to C19 steroids" (PDF). Appl. Environ. Microbiol. 32 (2): 310–1. PMC 170056. PMID 987752.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wang F-Q, Yao K, Wei D-Z. "From Soybean Phytosterols to Steroid Hormones, Soybean and Health". In El-Shemy H (ed.). Soybean and Health. InTech. doi:10.5772/18808. ISBN 978-953-307-535-8.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ "The Nobel Prize in Chemistry 1927". The Nobel Foundation.
- ^ "The Nobel Prize in Chemistry 1928". The Nobel Foundation.
- ^ "The Nobel Prize in Chemistry 1939". The Nobel Foundation.
- ^ "The Nobel Prize in Physiology or Medicine 1950". The Nobel Foundation.
- ^ "The Nobel Prize in Chemistry 1965". The Nobel Foundation.
- ^ "The Nobel Prize in Chemistry 1969". The Nobel Foundation.
- ^ "The Nobel Prize in Chemistry 1975". The Nobel Foundation.
Further reading
- Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. doi:10.1002/9780470973639. ISBN 978-0-470-66085-0.
- Yoder RA, Johnston JN (December 2005). "A case study in biomimetic total synthesis: polyolefin carbocyclizations to terpenes and steroids". Chem. Rev. 105 (12): 4730–56. doi:10.1021/cr040623. PMC 2575671. PMID 16351060. – review of the history of steroid synthesis, especially biomimetic
- Han, Thang S. (17 December 2013). "Treatment and health outcomes in adults with congenital adrenal hyperplasia". Nature Reviews Endocrinology. 10 (2): 115–124. doi:10.1038/nrendo.2013.239. PMID 24342885Figure 2: The adrenal steroidogenesis pathway.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: postscript (link)
External links
- Moss GP. "The Nomenclature of Steroids Home Page". Queen Mary University of London.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Bowen RA (October 20, 2001). "Steroidogenesis". Pathophysiology of the Endocrine System. Colorado State University.
- Heasley BH. "Modern Steroid Science". Steroid Blog. Google+.
