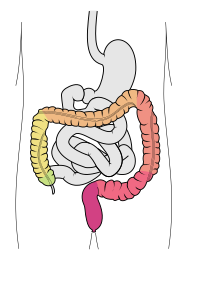
Large intestine
| Large intestine | |
|---|---|
 | |
| Details | |
| Part of | Gastrointestinal tract |
| System | Digestive system |
| Artery | Superior mesenteric, inferior mesenteric and iliac arteries |
| Vein | Superior and inferior mesenteric vein |
| Lymph | Inferior mesenteric lymph nodes |
| Identifiers | |
| Latin | colon or intestinum crassum |
| MeSH | D007420 |
| TA98 | A05.7.01.001 |
| TA2 | 2963 |
| FMA | 7201 |
| Anatomical terminology | |
 |
| Major parts of the |
| Gastrointestinal tract |
|---|
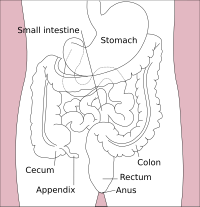
The large intestine, also known as the large bowel, is the last part of the gastrointestinal tract and of the digestive system in tetrapods. Water is absorbed here and the remaining waste material is stored in the rectum as feces before being removed by defecation.[1] The colon (progressing from the ascending colon to the transverse, the descending and finally the sigmoid colon) is the longest portion of the large intestine, and the terms "large intestine" and "colon" are often used interchangeably, but most sources define the large intestine as the combination of the cecum, colon, rectum, and anal canal.[1][2][3] Some other sources exclude the anal canal.[4][5][6]
In humans, the large intestine begins in the right iliac region of the pelvis, just at or below the waist, where it is joined to the end of the small intestine at the cecum, via the ileocecal valve. It then continues as the colon ascending the abdomen, across the width of the abdominal cavity as the transverse colon, and then descending to the rectum and its endpoint at the anal canal.[7] Overall, in humans, the large intestine is about 1.5 metres (5 ft) long, which is about one-fifth of the whole length of the human gastrointestinal tract.[8]
Structure
[edit]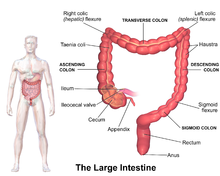
The colon of the large intestine is the last part of the digestive system. It has a segmented appearance due to a series of saccules called haustra.[9] It extracts water and salt from solid wastes before they are eliminated from the body and is the site in which the fermentation of unabsorbed material by the gut microbiota occurs. Unlike the small intestine, the colon does not play a major role in absorption of foods and nutrients. About 1.5 litres or 45 ounces of water arrives in the colon each day.[10]
The colon is the longest part of the large intestine and its average length in the adult human is 65 inches or 166 cm (range of 80 to 313 cm) for males, and 61 inches or 155 cm (range of 80 to 214 cm) for females.[11]
Sections
[edit]
In mammals, the large intestine consists of the cecum (including the appendix), colon (the longest part), rectum, and anal canal.[1]
The four sections of the colon are: the ascending colon, transverse colon, descending colon, and sigmoid colon. These sections turn at the colic flexures.
The parts of the colon are either intraperitoneal or behind it in the retroperitoneum. Retroperitoneal organs, in general, do not have a complete covering of peritoneum, so they are fixed in location. Intraperitoneal organs are completely surrounded by peritoneum and are therefore mobile.[12] Of the colon, the ascending colon, descending colon and rectum are retroperitoneal, while the cecum, appendix, transverse colon and sigmoid colon are intraperitoneal.[13] This is important as it affects which organs can be easily accessed during surgery, such as a laparotomy.
In terms of diameter, the cecum is the widest, averaging slightly less than 9 cm in healthy individuals, and the transverse colon averages less than 6 cm in diameter.[14] The descending and sigmoid colon are slightly smaller, with the sigmoid colon averaging 4–5 cm (1.6–2.0 in) in diameter.[14][15] Diameters larger than certain thresholds for each colonic section can be diagnostic for megacolon.
Cecum and appendix
[edit]The cecum is the first section of the large intestine and is involved in digestion, while the appendix which develops embryologically from it, is not involved in digestion and is considered to be part of the gut-associated lymphoid tissue. The function of the appendix is uncertain, but some sources believe that it has a role in housing a sample of the gut microbiota, and is able to help to repopulate the colon with microbiota if depleted during the course of an immune reaction. The appendix has also been shown to have a high concentration of lymphatic cells.
Ascending colon
[edit]The ascending colon is the first of four main sections of the large intestine. It is connected to the small intestine by a section of bowel called the cecum. The ascending colon runs upwards through the abdominal cavity toward the transverse colon for approximately eight inches (20 cm).
One of the main functions of the colon is to remove the water and other key nutrients from waste material and recycle it. As the waste material exits the small intestine through the ileocecal valve, it will move into the cecum and then to the ascending colon where this process of extraction starts. The waste material is pumped upwards toward the transverse colon by peristalsis. The ascending colon is sometimes attached to the appendix via Gerlach's valve. In ruminants, the ascending colon is known as the spiral colon.[16][17][18] Taking into account all ages and sexes, colon cancer occurs here most often (41%).[19]
Transverse colon
[edit]The transverse colon is the part of the colon from the hepatic flexure, also known as the right colic, (the turn of the colon by the liver) to the splenic flexure also known as the left colic, (the turn of the colon by the spleen). The transverse colon hangs off the stomach, attached to it by a large fold of peritoneum called the greater omentum. On the posterior side, the transverse colon is connected to the posterior abdominal wall by a mesentery known as the transverse mesocolon.
The transverse colon is encased in peritoneum, and is therefore mobile (unlike the parts of the colon immediately before and after it).
The proximal two-thirds of the transverse colon is perfused by the middle colic artery, a branch of the superior mesenteric artery (SMA), while the latter third is supplied by branches of the inferior mesenteric artery (IMA). The "watershed" area between these two blood supplies, which represents the embryologic division between the midgut and hindgut, is an area sensitive to ischemia.
Descending colon
[edit]The descending colon is the part of the colon from the splenic flexure to the beginning of the sigmoid colon. One function of the descending colon in the digestive system is to store feces that will be emptied into the rectum. It is retroperitoneal in two-thirds of humans. In the other third, it has a (usually short) mesentery.[20] The arterial supply comes via the left colic artery. The descending colon is also called the distal gut, as it is further along the gastrointestinal tract than the proximal gut. Gut flora are very dense in this region.
Sigmoid colon
[edit]The sigmoid colon is the part of the large intestine after the descending colon and before the rectum. The name sigmoid means S-shaped (see sigmoid; cf. sigmoid sinus). The walls of the sigmoid colon are muscular and contract to increase the pressure inside the colon, causing the stool to move into the rectum.
The sigmoid colon is supplied with blood from several branches (usually between 2 and 6) of the sigmoid arteries, a branch of the IMA. The IMA terminates as the superior rectal artery.
Sigmoidoscopy is a common diagnostic technique used to examine the sigmoid colon.
Rectum
[edit]The rectum is the last section of the large intestine. It holds the formed feces awaiting elimination via defecation. It is about 12 cm long.[21]
Appearance
[edit]The cecum – the first part of the large intestine
- Taeniae coli – three bands of smooth muscle
- Haustra – bulges caused by contraction of taeniae coli
- Epiploic appendages – small fat accumulations on the viscera
The taenia coli run the length of the large intestine. Because the taenia coli are shorter than the large bowel itself, the colon becomes sacculated, forming the haustra of the colon which are the shelf-like intraluminal projections.[22]
Blood supply
[edit]Arterial supply to the colon comes from branches of the superior mesenteric artery (SMA) and inferior mesenteric artery (IMA). Flow between these two systems communicates via the marginal artery of the colon that runs parallel to the colon for its entire length. Historically, a structure variously identified as the arc of Riolan or meandering mesenteric artery (of Moskowitz) was thought to connect the proximal SMA to the proximal IMA. This variably present structure would be important if either vessel were occluded. However, at least one review of the literature questions the existence of this vessel, with some experts calling for the abolition of these terms from future medical literature.[23]
Venous drainage usually mirrors colonic arterial supply, with the inferior mesenteric vein draining into the splenic vein, and the superior mesenteric vein joining the splenic vein to form the hepatic portal vein that then enters the liver. Middle rectal veins are an exception, delivering blood to inferior vena cava and bypassing the liver.[24]
Lymphatic drainage
[edit]Lymphatic drainage from the ascending colon and proximal two-thirds of the transverse colon is to the ileocolic lymph nodes and the superior mesenteric lymph nodes, which drain into the cisterna chyli.[25] The lymph from the distal one-third of the transverse colon, the descending colon, the sigmoid colon, and the upper rectum drain into the inferior mesenteric and colic lymph nodes.[25] The lower rectum to the anal canal above the pectinate line drain to the internal ileocolic nodes.[26] The anal canal below the pectinate line drains into the superficial inguinal nodes.[26] The pectinate line only roughly marks this transition.
Nerve supply
[edit]Sympathetic supply: superior & inferior mesenteric ganglia; parasympathetic supply: vagus & sacral plexus (S2-S4)[citation needed]
Development
[edit]The endoderm, mesoderm and ectoderm are germ layers that develop in a process called gastrulation. Gastrulation occurs early in human development. The gastrointestinal tract is derived from these layers.[27]
Variation
[edit]One variation on the normal anatomy of the colon occurs when extra loops form, resulting in a colon that is up to five metres longer than normal. This condition, referred to as redundant colon, typically has no direct major health consequences, though rarely volvulus occurs, resulting in obstruction and requiring immediate medical attention.[28][29] A significant indirect health consequence is that use of a standard adult colonoscope is difficult and in some cases impossible when a redundant colon is present, though specialized variants on the instrument (including the pediatric variant) are useful in overcoming this problem.[30]
Microanatomy
[edit]Colonic crypts
[edit]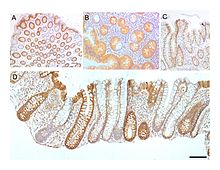
The wall of the large intestine is lined with simple columnar epithelium with invaginations. The invaginations are called the intestinal glands or colonic crypts.
-
Micrograph of normal large instestinal crypts.
-
Anatomy of normal large intestinal crypts
The colon crypts are shaped like microscopic thick walled test tubes with a central hole down the length of the tube (the crypt lumen). Four tissue sections are shown here, two cut across the long axes of the crypts and two cut parallel to the long axes. In these images the cells have been stained by immunohistochemistry to show a brown-orange color if the cells produce a mitochondrial protein called cytochrome c oxidase subunit I (CCOI). The nuclei of the cells (located at the outer edges of the cells lining the walls of the crypts) are stained blue-gray with haematoxylin. As seen in panels C and D, crypts are about 75 to about 110 cells long. Baker et al.[32] found that the average crypt circumference is 23 cells. Thus, by the images shown here, there are an average of about 1,725 to 2,530 cells per colonic crypt. Nooteboom et al.[33] measuring the number of cells in a small number of crypts reported a range of 1,500 to 4,900 cells per colonic crypt. Cells are produced at the crypt base and migrate upward along the crypt axis before being shed into the colonic lumen days later.[32] There are 5 to 6 stem cells at the bases of the crypts.[32]
As estimated from the image in panel A, there are about 100 colonic crypts per square millimeter of the colonic epithelium.[34] Since the average length of the human colon is 160.5 cm[11] and the average inner circumference of the colon is 6.2 cm,[34] the inner surface epithelial area of the human colon has an average area of about 995 cm2, which includes 9,950,000 (close to 10 million) crypts.
In the four tissue sections shown here, many of the intestinal glands have cells with a mitochondrial DNA mutation in the CCOI gene and appear mostly white, with their main color being the blue-gray staining of the nuclei. As seen in panel B, a portion of the stem cells of three crypts appear to have a mutation in CCOI, so that 40% to 50% of the cells arising from those stem cells form a white segment in the cross cut area.
Overall, the percent of crypts deficient for CCOI is less than 1% before age 40, but then increases linearly with age.[31] Colonic crypts deficient for CCOI in women reaches, on average, 18% in women and 23% in men by 80–84 years of age.[31]
Crypts of the colon can reproduce by fission, as seen in panel C, where a crypt is fissioning to form two crypts, and in panel B where at least one crypt appears to be fissioning. Most crypts deficient in CCOI are in clusters of crypts (clones of crypts) with two or more CCOI-deficient crypts adjacent to each other (see panel D).[31]
Mucosa
[edit]About 150 of the many thousands of protein coding genes expressed in the large intestine, some are specific to the mucous membrane in different regions and include CEACAM7.[35]
Function
[edit]
The large intestine absorbs water and any remaining absorbable nutrients from the food before sending the indigestible matter to the rectum. The colon absorbs vitamins that are created by the colonic bacteria, such as thiamine, riboflavin, and vitamin K (especially important as the daily ingestion of vitamin K is not normally enough to maintain adequate blood coagulation).[36][citation needed][37] It also compacts feces, and stores fecal matter in the rectum until it can be discharged via the anus in defecation.
The large intestine also secretes K+ and Cl-. Chloride secretion increases in cystic fibrosis. Recycling of various nutrients takes place in the colon. Examples include fermentation of carbohydrates, short chain fatty acids, and urea cycling.[38][citation needed]
The appendix contains a small amount of mucosa-associated lymphoid tissue which gives the appendix an undetermined role in immunity. However, the appendix is known to be important in fetal life as it contains endocrine cells that release biogenic amines and peptide hormones important for homeostasis during early growth and development.[39]
By the time the chyme has reached this tube, most nutrients and 90% of the water have been absorbed by the body. Indeed, as demonstrated by the commonality of ileostomy procedures, it is possible for many people to live without large portions of their large intestine, or even without it completely. At this point only some electrolytes like sodium, magnesium, and chloride are left as well as indigestible parts of ingested food (e.g., a large part of ingested amylose, starch which has been shielded from digestion heretofore, and dietary fiber, which is largely indigestible carbohydrate in either soluble or insoluble form). As the chyme moves through the large intestine, most of the remaining water is removed, while the chyme is mixed with mucus and bacteria (known as gut flora), and becomes feces. The ascending colon receives fecal material as a liquid. The muscles of the colon then move the watery waste material forward and slowly absorb all the excess water, causing the stools to gradually solidify as they move along into the descending colon.[40]
The bacteria break down some of the fiber for their own nourishment and create acetate, propionate, and butyrate as waste products, which in turn are used by the cell lining of the colon for nourishment.[41] No protein is made available. In humans, perhaps 10% of the undigested carbohydrate thus becomes available, though this may vary with diet;[42] in other animals, including other apes and primates, who have proportionally larger colons, more is made available, thus permitting a higher portion of plant material in the diet. The large intestine[43] produces no digestive enzymes — chemical digestion is completed in the small intestine before the chyme reaches the large intestine. The pH in the colon varies between 5.5 and 7 (slightly acidic to neutral).[44]
Standing gradient osmosis
[edit]Water absorption at the colon typically proceeds against a transmucosal osmotic pressure gradient. The standing gradient osmosis is the reabsorption of water against the osmotic gradient in the intestines. Cells occupying the intestinal lining pump sodium ions into the intercellular space, raising the osmolarity of the intercellular fluid. This hypertonic fluid creates an osmotic pressure that drives water into the lateral intercellular spaces by osmosis via tight junctions and adjacent cells, which then in turn moves across the basement membrane and into the capillaries, while more sodium ions are pumped again into the intercellular fluid.[45] Although water travels down an osmotic gradient in each individual step, overall, water usually travels against the osmotic gradient due to the pumping of sodium ions into the intercellular fluid. This allows the large intestine to absorb water despite the blood in capillaries being hypotonic compared to the fluid within the intestinal lumen.
Gut flora
[edit]The large intestine houses over 700 species of bacteria that perform a variety of functions, as well as fungi, protozoa, and archaea. Species diversity varies by geography and diet.[46] The microbes in a human distal gut often number in the vicinity of 100 trillion, and can weigh around 200 grams (0.44 pounds). This mass of mostly symbiotic microbes has recently been called the latest human organ to be "discovered" or in other words, the "forgotten organ".[47]
The large intestine absorbs some of the products formed by the bacteria inhabiting this region. Undigested polysaccharides (fiber) are metabolized to short-chain fatty acids by bacteria in the large intestine and absorbed by passive diffusion. The bicarbonate that the large intestine secretes helps to neutralize the increased acidity resulting from the formation of these fatty acids.[48]
These bacteria also produce large amounts of vitamins, especially vitamin K and biotin (a B vitamin), for absorption into the blood. Although this source of vitamins, in general, provides only a small part of the daily requirement, it makes a significant contribution when dietary vitamin intake is low. An individual who depends on absorption of vitamins formed by bacteria in the large intestine may become vitamin-deficient if treated with antibiotics that inhibit the vitamin producing species of bacteria as well as the intended disease-causing bacteria.[49]
Other bacterial products include gas (flatus), which is a mixture of nitrogen and carbon dioxide, with small amounts of the gases hydrogen, methane, and hydrogen sulfide. Bacterial fermentation of undigested polysaccharides produces these. Some of the fecal odor is due to indoles, metabolized from the amino acid tryptophan. The normal flora is also essential in the development of certain tissues, including the cecum and lymphatics.[citation needed]
They are also involved in the production of cross-reactive antibodies. These are antibodies produced by the immune system against the normal flora, that are also effective against related pathogens, thereby preventing infection or invasion.
The two most prevalent phyla of the colon are Bacillota and Bacteroidota. The ratio between the two seems to vary widely as reported by the Human Microbiome Project.[50] Bacteroides are implicated in the initiation of colitis and colon cancer. Bifidobacteria are also abundant, and are often described as 'friendly bacteria'.[51][52]
A mucus layer protects the large intestine from attacks from colonic commensal bacteria.[53]
Clinical significance
[edit]Disease
[edit]Following are the most common diseases or disorders of the colon:
- Angiodysplasia of the colon
- Appendicitis
- Chronic functional abdominal pain
- Colitis
- Colorectal cancer
- Colorectal polyp
- Constipation
- Crohn's disease
- Diarrhea
- Diverticulitis
- Diverticulosis
- Hirschsprung's disease (aganglionosis)
- Ileus
- Intussusception
- Irritable bowel syndrome
- Pseudomembranous colitis
- Ulcerative colitis and toxic megacolon
Colonoscopy
[edit]
normal mucosa. The spleen can be seen through it
Colonoscopy is the endoscopic examination of the large intestine and the distal part of the small bowel with a CCD camera or a fiber optic camera on a flexible tube passed through the anus. It can provide a visual diagnosis (e.g. ulceration, polyps) and grants the opportunity for biopsy or removal of suspected colorectal cancer lesions. Colonoscopy can remove polyps as small as one millimetre or less. Once polyps are removed, they can be studied with the aid of a microscope to determine if they are precancerous or not. It takes 15 years or fewer for a polyp to turn cancerous.
Colonoscopy is similar to sigmoidoscopy—the difference being related to which parts of the colon each can examine. A colonoscopy allows an examination of the entire colon (1200–1500 mm in length). A sigmoidoscopy allows an examination of the distal portion (about 600 mm) of the colon, which may be sufficient because benefits to cancer survival of colonoscopy have been limited to the detection of lesions in the distal portion of the colon.[54][55][56]
A sigmoidoscopy is often used as a screening procedure for a full colonoscopy, often done in conjunction with a stool-based test such as a fecal occult blood test (FOBT), fecal immunochemical test (FIT), or multi-target stool DNA test (Cologuard) or blood-based test, SEPT9 DNA methylation test (Epi proColon).[57] About 5% of these screened patients are referred to colonoscopy.[58]
Virtual colonoscopy, which uses 2D and 3D imagery reconstructed from computed tomography (CT) scans or from nuclear magnetic resonance (MR) scans, is also possible, as a totally non-invasive medical test, although it is not standard and still under investigation regarding its diagnostic abilities. Furthermore, virtual colonoscopy does not allow for therapeutic maneuvers such as polyp/tumour removal or biopsy nor visualization of lesions smaller than 5 millimeters. If a growth or polyp is detected using CT colonography, a standard colonoscopy would still need to be performed. Additionally, surgeons have lately been using the term pouchoscopy to refer to a colonoscopy of the ileo-anal pouch.
Other animals
[edit]The large intestine is truly distinct only in tetrapods, in which it is almost always separated from the small intestine by an ileocaecal valve. In most vertebrates, however, it is a relatively short structure running directly to the anus, although noticeably wider than the small intestine. Although the caecum is present in most amniotes, only in mammals does the remainder of the large intestine develop into a true colon.[59]
In some small mammals, the colon is straight, as it is in other tetrapods, but, in the majority of mammalian species, it is divided into ascending and descending portions; a distinct transverse colon is typically present only in primates. However, the taeniae coli and accompanying haustra are not found in either carnivorans or ruminants. The rectum of mammals (other than monotremes) is derived from the cloaca of other vertebrates, and is, therefore, not truly homologous with the "rectum" found in these species.[59]
In some fish, there is no true large intestine, but simply a short rectum connecting the end of the digestive part of the gut to the cloaca. In sharks, this includes a rectal gland that secretes salt to help the animal maintain osmotic balance with the seawater. The gland somewhat resembles a caecum in structure but is not a homologous structure.[59]
Additional images
[edit]-
Intestines
-
Colon. Deep dissection. Anterior view.
See also
[edit]References
[edit]![]() This article incorporates text in the public domain from page 1177 of the 20th edition of Gray's Anatomy (1918)
This article incorporates text in the public domain from page 1177 of the 20th edition of Gray's Anatomy (1918)
- ^ a b c "large intestine". NCI Dictionary of Cancer Terms. National Cancer Institute, National Institutes of Health. 2011-02-02. Retrieved 2014-03-04.
- ^ Kapoor, Vinay Kumar (13 Jul 2011). Gest, Thomas R. (ed.). "Large Intestine Anatomy". Medscape. WebMD LLC. Retrieved 2013-08-20.
- ^ Gray, Henry (1918). Gray's Anatomy. Philadelphia: Lea & Febiger.
- ^ "large intestine". Mosby's Medical Dictionary (8th ed.). Elsevier. 2009. ISBN 9780323052900.
- ^ "intestine". Concise Medical Dictionary. Oxford University Press. 2010. ISBN 9780199557141.
- ^ "large intestine". A Dictionary of Biology. Oxford University Press. 2013. ISBN 9780199204625.
- ^ "Large intestine". Archived from the original on 2015-08-28. Retrieved 2016-07-24.
- ^ Drake, R.L.; Vogl, W.; Mitchell, A.W.M. (2010). Gray's Anatomy for Students. Philadelphia: Churchill Livingstone.
- ^ Azzouz, Laura; Sharma, Sandeep (2020). "Physiology, Large Intestine". NCBI Bookshelf. PMID 29939634.
- ^ David Krogh (2010), Biology: A Guide to the Natural World, Benjamin-Cummings Publishing Company, p. 597, ISBN 978-0-321-61655-5
- ^ a b Hounnou G, Destrieux C, Desmé J, Bertrand P, Velut S (2002). "Anatomical study of the length of the human intestine". Surg Radiol Anat. 24 (5): 290–294. doi:10.1007/s00276-002-0057-y. PMID 12497219. S2CID 33366428.
- ^ "Peritoneum". Mananatomy.com. 2013-01-18. Archived from the original on 2018-10-08. Retrieved 2013-02-07.
- ^ "Untitled".
- ^ a b Horton, K. M.; Corl, F. M.; Fishman, E. K. (March 2000). "CT evaluation of the colon: inflammatory disease". Radiographics. 20 (2): 399–418. doi:10.1148/radiographics.20.2.g00mc15399. ISSN 0271-5333. PMID 10715339.
- ^ Rossini, Francesco Paolo (1975), "The normal colon", in Rossini, Francesco Paolo (ed.), Atlas of coloscopy, Springer New York, pp. 46–55, doi:10.1007/978-1-4615-9650-9_12, ISBN 9781461596509
- ^ Medical dictionary
- ^ Spiral colon and caecum, archived from the original on 2016-03-04, retrieved 2014-04-02
- ^ "Answers – The Most Trusted Place for Answering Life's Questions". Answers.com.
- ^ Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A, Jemal A (March 1, 2017). "Colorectal cancer statistics". CA Cancer J. Clin. 67 (3): 177–193. doi:10.3322/caac.21395. PMID 28248415.
- ^ Smithivas, T.; Hyams, P. J.; Rahal, J. J. (1971-12-01). "Gentamicin and ampicillin in human bile". The Journal of Infectious Diseases. 124 Suppl: S106–108. doi:10.1093/infdis/124.supplement_1.s106. ISSN 0022-1899. PMID 5126238.
- ^ "Anatomy of Colon and Rectum | SEER Training". training.seer.cancer.gov. Retrieved 2021-04-14.
- ^ Anatomy at a Glance by Omar Faiz and David Moffat
- ^ Lange, Johan F.; Komen, Niels; Akkerman, Germaine; Nout, Erik; Horstmanshoff, Herman; Schlesinger, Frans; Bonjer, Jaap; Kleinrensink, Gerrit-Jan (June 2007). "Riolan's arch: confusing, misnomer, and obsolete. A literature survey of the connection(s) between the superior and inferior mesenteric arteries". Am J Surg. 193 (6): 742–748. doi:10.1016/j.amjsurg.2006.10.022. PMID 17512289.
- ^ van Hoogdalem, Edward; de Boer, Albertus G.; Breimer, Douwe D. (July 1991). "Pharmacokinetics of rectal drug administration, Part I. General considerations and clinical applications of centrally acting drugs". Clinical Pharmacokinetics. 21 (1): 14. doi:10.2165/00003088-199121010-00002. ISSN 0312-5963. Retrieved 18 March 2024.
The superior rectal vein, perfusing the upper part of the rectum, drains into the portal vein and subsequently into the liver On the other hand, the middle and inferior rectal veins drain the lower part of the rectum and venous blood is returned to the inferior vena cava.
- ^ a b Snell, Richard S. (1992). Clinical Anatomy for Medical Students (4th ed.). Boston: Little, Brown, and Company. pp. 53–54.
- ^ a b Le, Tao; et al. (2014). First Aid for the USMLE Step 1. McGraw-Hill Education. p. 196.
- ^ Wilson, Danielle J.; Bordoni, Bruno (2022), "Embryology, Bowel", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 31424831, retrieved 2022-05-27
- ^ Mayo Clinic Staff (2006-10-13). "Redundant colon: A health concern?". Ask a Digestive System Specialist. MayoClinic.com. Archived from the original on 2007-09-29. Retrieved 2007-06-11.
- ^ Mayo Clinic Staff. "Redundant colon: A health concern? (Above with active image links)". riversideonline.com. Archived from the original on 9 November 2013. Retrieved 8 November 2013.
- ^ Lichtenstein, Gary R.; Peter D. Park; William B. Long; Gregory G. Ginsberg; Michael L. Kochman (18 August 1998). "Use of a Push Enteroscope Improves Ability to Perform Total Colonoscopy in Previously Unsuccessful Attempts at Colonoscopy in Adult Patients". The American Journal of Gastroenterology. 94 (1): 187–190. doi:10.1111/j.1572-0241.1999.00794.x. PMID 9934753. S2CID 24536782. Note: single use PDF copy provided free by Blackwell Publishing for purposes of Wikipedia content enrichment.
- ^ a b c d Bernstein C, Facista A, Nguyen H, Zaitlin B, Hassounah N, Loustaunau C, Payne CM, Banerjee B, Goldschmid S, Tsikitis VL, Krouse R, Bernstein H (2010). "Cancer and age related colonic crypt deficiencies in cytochrome c oxidase I". World J Gastrointest Oncol. 2 (12): 429–442. doi:10.4251/wjgo.v2.i12.429. PMC 3011097. PMID 21191537.
- ^ a b c Baker AM, Cereser B, Melton S, Fletcher AG, Rodriguez-Justo M, Tadrous PJ, Humphries A, Elia G, McDonald SA, Wright NA, Simons BD, Jansen M, Graham TA (2014). "Quantification of crypt and stem cell evolution in the normal and neoplastic human colon". Cell Rep. 8 (4): 940–947. doi:10.1016/j.celrep.2014.07.019. PMC 4471679. PMID 25127143.
- ^ Nooteboom M, Johnson R, Taylor RW, Wright NA, Lightowlers RN, Kirkwood TB, Mathers JC, Turnbull DM, Greaves LC (2010). "Age-associated mitochondrial DNA mutations lead to small but significant changes in cell proliferation and apoptosis in human colonic crypts". Aging Cell. 9 (1): 96–99. doi:10.1111/j.1474-9726.2009.00531.x. PMC 2816353. PMID 19878146.
- ^ a b Nguyen H, Loustaunau C, Facista A, Ramsey L, Hassounah N, Taylor H, Krouse R, Payne CM, Tsikitis VL, Goldschmid S, Banerjee B, Perini RF, Bernstein C (2010). "Deficient Pms2, ERCC1, Ku86, CcOI in field defects during progression to colon cancer". J Vis Exp (41). doi:10.3791/1931. PMC 3149991. PMID 20689513.
- ^ Gremel, Gabriela; Wanders, Alkwin; Cedernaes, Jonathan; Fagerberg, Linn; Hallström, Björn; Edlund, Karolina; Sjöstedt, Evelina; Uhlén, Mathias; Pontén, Fredrik (2015-01-01). "The human gastrointestinal tract-specific transcriptome and proteome as defined by RNA sequencing and antibody-based profiling". Journal of Gastroenterology. 50 (1): 46–57. doi:10.1007/s00535-014-0958-7. ISSN 0944-1174. PMID 24789573. S2CID 21302849.
- ^ Sellers, Rani S.; Morton, Daniel (2014). "The Colon: From Banal to Brilliant". Toxicologic Pathology. 42 (1): 67–81. doi:10.1177/0192623313505930. PMID 24129758. S2CID 20465985.
- ^ Booth, Sarah (April 2012). "Vitamin K: Food Consumption and Dietary Intakes". Food Nutrition Research. 56. doi:10.3402/fnr.v56i0.5505. PMC 3321250. PMID 22489217.
- ^ "The Large Intestine (Human)". News-Medical.net. 2009-11-17. Retrieved 2017-03-15.
- ^ Martin, Loren G. (1999-10-21). "What is the function of the human appendix? Did it once have a purpose that has since been lost?". Scientific American. Retrieved 2014-03-03.
- ^ La función de la hidroterapia de colon Retrieved on 2010-01-21
- ^ Terry L. Miller; Meyer J. Wolin (1996). "Pathways of Acetate, Propionate, and Butyrate Formation by the Human Fecal Microbial Flora". Applied and Environmental Microbiology. 62 (5): 1589–1592. Bibcode:1996ApEnM..62.1589M. doi:10.1128/AEM.62.5.1589-1592.1996. PMC 167932. PMID 8633856.
- ^ McNeil, NI (1984). "The contribution of the large intestine to energy supplies in man". The American Journal of Clinical Nutrition. 39 (2): 338–342. doi:10.1093/ajcn/39.2.338. PMID 6320630.
- ^ lorriben (2016-07-09). "What Side is Your Appendix Located?". Maglenia. Archived from the original on 2016-10-09. Retrieved 2016-10-23.
- ^ Function Of The Large Intestine Archived 2013-11-05 at the Wayback Machine Retrieved on 2010-01-21
- ^ "Absorption of Water and Electrolytes".
- ^ Yatsunenko, Tanya; et al. (2012). "Human gut microbiome viewed across age and geography". Nature. 486 (7402): 222–227. Bibcode:2012Natur.486..222Y. doi:10.1038/nature11053. PMC 3376388. PMID 22699611.
- ^ O'Hara, Ann M.; Shanahan, Fergus (2006). "The gut flora as a forgotten organ". EMBO Reports. 7 (7): 688–693. doi:10.1038/sj.embor.7400731. PMC 1500832. PMID 16819463.
- ^ den Besten, Gijs; van Eunen, Karen; Groen, Albert K.; Venema, Koen; Reijngoud, Dirk-Jan; Bakker, Barbara M. (2013-09-01). "The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism". Journal of Lipid Research. 54 (9): 2325–2340. doi:10.1194/jlr.R036012. ISSN 0022-2275. PMC 3735932. PMID 23821742.
- ^ Murdoch, Travis B.; Detsky, Allan S. (2012-12-01). "Time to Recognize Our Fellow Travellers". Journal of General Internal Medicine. 27 (12): 1704–1706. doi:10.1007/s11606-012-2105-6. ISSN 0884-8734. PMC 3509308. PMID 22588826.
- ^ Human Microbiome Project Consortium (Jun 14, 2012). "Structure, function and diversity of the healthy human microbiome". Nature. 486 (7402): 207–214. Bibcode:2012Natur.486..207T. doi:10.1038/nature11234. PMC 3564958. PMID 22699609.
- ^ Bloom, Seth M.; Bijanki, Vinieth N.; Nava, Gerardo M.; Sun, Lulu; Malvin, Nicole P.; Donermeyer, David L.; Dunne, W. Michael; Allen, Paul M.; Stappenbeck, Thaddeus S. (2011-05-19). "Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease". Cell Host & Microbe. 9 (5): 390–403. doi:10.1016/j.chom.2011.04.009. ISSN 1931-3128. PMC 3241010. PMID 21575910.
- ^ Bottacini, Francesca; Ventura, Marco; van Sinderen, Douwe; O'Connell Motherway, Mary (2014-08-29). "Diversity, ecology and intestinal function of bifidobacteria". Microbial Cell Factories. 13 (Suppl 1): S4. doi:10.1186/1475-2859-13-S1-S4. ISSN 1475-2859. PMC 4155821. PMID 25186128.
- ^ Johansson, Malin E.V.; Sjövall, Henrik; Hansson, Gunnar C. (2013-06-01). "The gastrointestinal mucus system in health and disease". Nature Reviews. Gastroenterology & Hepatology. 10 (6): 352–361. doi:10.1038/nrgastro.2013.35. ISSN 1759-5045. PMC 3758667. PMID 23478383.
- ^ Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L (January 2009). "Association of colonoscopy and death from colorectal cancer". Ann. Intern. Med. 150 (1): 1–8. doi:10.7326/0003-4819-150-1-200901060-00306. PMID 19075198. S2CID 24130424. as PDF Archived 2012-01-18 at the Wayback Machine
- ^ Singh H, Nugent Z, Mahmud SM, Demers AA, Bernstein CN (March 2010). "Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies". Am J Gastroenterol. 105 (3): 663–673. doi:10.1038/ajg.2009.650. PMID 19904239. S2CID 11145247.
- ^ Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Alterhofen L, Haug U (January 2010). "Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study". J Natl Cancer Inst. 102 (2): 89–95. doi:10.1093/jnci/djp436. PMID 20042716. S2CID 1887714.
- ^ Tepus, M; Yau, TO (20 May 2020). "Non-Invasive Colorectal Cancer Screening: An Overview". Gastrointestinal Tumors. 7 (3): 62–73. doi:10.1159/000507701. PMC 7445682. PMID 32903904.
- ^ Atkin WS, Edwards R, Kralj-Hans I, et al. (May 2010). "Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial". Lancet. 375 (9726): 1624–33. doi:10.1016/S0140-6736(10)60551-X. PMID 20430429. S2CID 15194212. as PDF Archived 2012-03-24 at the Wayback Machine
- ^ a b c Romer, Alfred Sherwood; Parsons, Thomas S. (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. pp. 351–354. ISBN 978-0-03-910284-5.
External links
[edit]- 09-118h. at Merck Manual of Diagnosis and Therapy Home Edition




