Ribosomal DNA
The ribosomal DNA (abbreviated rDNA) consists of a group of ribosomal RNA encoding genes and related regulatory elements, and is widespread in similar configuration in all domains of life. The ribosomal DNA encodes the non-coding ribosomal RNA, integral structural elements in the assembly of ribosomes, its importance making it the most abundant section of RNA found in cells of eukaryotes.[1] Additionally, these segments includes regulatory sections, such as a promotor specific to the RNA polymerase I, as well as both transcribed and non-transcribed spacer segments.
Due to their high importance in the assembly of ribosomes for protein biosynthesis, the rDNA genes are generally highly conserved in molecular evolution. The number of copies can vary considerably per species.[1] Ribosomal DNA is widely used for phylogenetic studies.[2][3]
Structure
[edit]| Type | SSU rRNA | LSU rRNA |
|---|---|---|
| Eukaryotes | 18S rRNA | 28S rRNA 5.8S rRNA 5S rRNA |
| Bacteria | 16S rRNA | 23S rRNA 5S rRNA |
| Mitochondrial | MT-RNR1 (12S rRNA) | MT-RNR2 (16S rRNA) |
| Plastid | 16S rRNA | 23S rRNA 4.5S rRNA 5S rRNA |
The ribosomal DNA includes all genes coding for the non-coding structural ribosomal RNA molecules. Across all domains of life, these are the structural sequences of the small subunit (16S or 18S rRNA) and the large subunit (23S or 28S rRNA). The assembly of the latter also include the 5S rRNA as well as the additional 5.8S rRNA in eukaryotes.
The rDNA-genes are commonly present with multiple copies in the genome, where they are organized in linked groups in most species, separated by an internal transcribed spacer (ITS) and preceded by the External transcribed spacer (ETS). The 5S rRNA is also linked to these rDNA region in prokaryotes, while it is located in separate repeating regions in most eukaryotes.[4] They are transcribed together to a precursor RNA which is then processed to equal amounts of each rRNA.
Prokaryotes
[edit]The primary structural rRNA molecules in Bacteria and Archaea are smaller than their counterparts in eukaryotes, grouped as 16S rRNA and 23S rRNA. Meanwhile, the 5S rRNA also present in prokaryotes, is of a similar size to eukaryotes.
A notable amount of bacteria and archaea diverge from the canonical structure of the operon containing the rDNA genes, carrying the "unlinked" genes in different places of their genome.[5]
Plastids
[edit]Ribosomal DNA in chloroplasts follows the structure of prokaryotic ribosomal DNA.
Eukaryotes
[edit]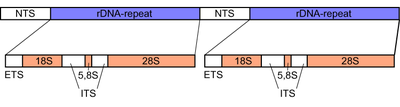

The rDNA gene cluster of eukaryotes consists of the genes for the 18S, 5.8S and 28S rRNA, separated by the two ITS-1 and ITS-2 spacers. The active genome of eukaryotes contains several hundred copies of the rDNA transcriptional unit as tandem repeats, they are organized in nucleolus organizer regions (NORs),[4] which in turn can be present at multiple loci in the genome.[6]
Similar to the structure of prokaryotes, the 5S rRNA is appended to the rDNA cluster in the Saccharomycetes (Hemiascomycetes)[6] such as Saccharomyces cerevisiae.[4] Most eukaryotes however, carry the gene for the 5S rRNA in separate gene repeats at different loci in the genome.[6][4]
5S rDNA is also present in independent tandem repeats as in Drosophila.[6] As repetitive DNA regions often undergo recombination events, the rDNA repeats have many regulatory mechanisms that keep the DNA from undergoing mutations,[example needed] thus keeping the rDNA conserved.[1]
In the nucleus, the nucleolus organizer regions give rise to the nucleolus, where the rDNA regions of the chromosome forms expanded chromosomal loops, accessible for transcription of rRNA. In rDNA, the tandem repeats are mostly found in the nucleolus; but heterochromatic rDNA is found outside of the nucleolus. However, transcriptionally active rDNA resides inside of the nucleolus itself.[1]
Humans
[edit]The human genome contains a total of 560 copies[4] of the rDNA transcriptional unit, spread across five chromosomes with nucleolus organizer regions. The repeat clusters are located on the acrocentric chromosomes 13 (RNR1), 14 (RNR2), 15 (RNR3), 21 (RNR4) and 22 (RNR5).[7]
Ciliates
[edit]In ciliates, the presence of a generative micronucleus next to the vegetative macronucleus allows for the reduction of rDNA genes in the germline. The exact number of copies in the micronucleus core genome ranging from several copies in Paramecium[8] as low as a single copy in Tetrahymena thermophila[4] and other Tetrahymena species. During macronucleus formation, the regions containing the rDNA gene clusters are amplified, dramatically increasing the amount of available templates for transcription up to several thousand copies. In some ciliate genera, such as Tetrahymena or the Hypotrich genus Oxytricha,[8] extensive fragmentation of the amplified DNA leads to the formation of microchromosomes, centered on the rDNA transcriptional unit.[8] Similar processes are reported from Glaucoma chattoni and to lesser extent from Paramecium.[8]
Sequence homogeneity
[edit]In the large rDNA array, polymorphisms between rDNA repeat units are very low, indicating that rDNA tandem arrays are evolving through concerted evolution.[6] However, the mechanism of concerted evolution is imperfect, such that polymorphisms between repeats within an individual can occur at significant levels and may confound phylogenetic analyses for closely related organisms.[9][10]
5S tandem repeat sequences in several Drosophila were compared with each other; the result revealed that insertions and deletions occurred frequently between species and often flanked by conserved sequences.[11] They could occur by slippage of the newly synthesized strand during DNA replication or by gene conversion.[11]
Sequence divergence
[edit]The rDNA transcription tracts have low rate of polymorphism among species, which allows interspecific comparison to elucidate phylogenetic relationship using only a few specimens. Coding regions of rDNA are highly conserved among species but ITS regions are variable due to insertions, deletions, and point mutations. Between remote species as human and frog comparison of sequences at ITS tracts is not appropriate.[12] Conserved sequences at coding regions of rDNA allow comparisons of remote species, even between yeast and human. Human 5.8S rRNA has 75% identity with yeast 5.8S rRNA.[13] In cases for sibling species, comparison of the rDNA segment including ITS tracts among species and phylogenetic analysis are made satisfactorily.[14][15] The different coding regions of the rDNA repeats usually show distinct evolutionary rates. As a result, this DNA can provide phylogenetic information of species belonging to wide systematic levels.[2]
Recombination-stimulating activity
[edit]A fragment of yeast rDNA containing the 5S gene, non-transcribed spacer DNA, and part of the 35S gene has localized cis-acting mitotic recombination stimulating activity.[16] This DNA fragment contains a mitotic recombination hotspot, referred to as HOT1. HOT1 expresses recombination-stimulating activity when it is inserted into novel locations in the yeast genome. HOT1 includes an RNA polymerase I (PolI) transcription promoter that catalyzes 35S ribosomal rRNA gene transcription. In a PolI defective mutant, the HOT1 hotspot recombination-stimulating activity is abolished. The level of PolI transcription in HOT1 appears to determine the level of recombination.[17]
Clinical significance
[edit]Diseases can be associated with DNA mutations where DNA can be expanded, such as Huntington's disease, or lost due to deletion mutations. The same is true for mutations that occur in rDNA repeats; it has been found that if the genes that are associated with the synthesis of ribosomes are disrupted or mutated, it can result in various diseases associated with the skeleton or bone marrow. Also, any damage or disruption to the enzymes that protect the tandem repeats of the rDNA, can result in lower synthesis of ribosomes, which also lead to other defects in the cell. Neurological diseases can also arise from mutations in the rDNA tandem repeats, such as Bloom syndrome, which occurs when the number of tandem repeats increases close to a hundred-fold; compared with that of the normal number of tandem repeats. Various types of cancers can also be born from mutations of the tandem repeats in the ribosomal DNA. Cell lines can become malignant from either a rearrangement of the tandem repeats, or an expansion of the repeats in the rDNA.[18]
References
[edit]- ^ a b c d Warmerdam, Daniël O.; Wolthuis, Rob M. F. (2019-03-01). "Keeping ribosomal DNA intact: a repeating challenge". Chromosome Research. 27 (1): 57–72. doi:10.1007/s10577-018-9594-z. ISSN 1573-6849. PMC 6394564. PMID 30556094.
- ^ a b Hillis DM, Dixon MT (December 1991). "Ribosomal DNA: Molecular Evolution and Phylogenetic Inference". The Quarterly Review of Biology. 66 (4): 411–53. doi:10.1086/417338. PMID 1784710. S2CID 32027097.
- ^ Weisburg WG, Barns SM, Pelletier DA, Lane DJ (January 1991). "16S ribosomal DNA amplification for phylogenetic study". Journal of Bacteriology. 173 (2): 697–703. doi:10.1128/jb.173.2.697-703.1991. PMC 207061. PMID 1987160.
- ^ a b c d e f Graw, Jochen (2015). Genetik [Genetics] (in German) (6th ed.). Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg. doi:10.1007/978-3-662-44817-5. ISBN 978-3-662-44816-8.
- ^ Brewer TE, Albertsen M, Edwards A, Kirkegaard RH, Rocha EP, Fierer N (February 2020). "Unlinked rRNA genes are widespread among bacteria and archaea". The ISME Journal. 14 (2): 597–608. Bibcode:2020ISMEJ..14..597B. doi:10.1038/s41396-019-0552-3. PMC 6976660. PMID 31712737.
- ^ a b c d e Richard GF, Kerrest A, Dujon B (December 2008). "Comparative genomics and molecular dynamics of DNA repeats in eukaryotes". Microbiology and Molecular Biology Reviews. 72 (4): 686–727. doi:10.1128/MMBR.00011-08. PMC 2593564. PMID 19052325.
- ^ Schmidt, Olaf (2017). Fritsche, Olaf (ed.). Genetik und Molekularbiologie [Genetics and molecular biology]. Kompaktwissen Biologie (in German). Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg. doi:10.1007/978-3-662-50274-7. ISBN 978-3-662-50273-0.
- ^ a b c d Prescott, David M. (June 1994). "The DNA of Ciliated Protozoa". Microbiological Reviews. 58 (2): 233–267. doi:10.1128/mr.58.2.233-267.1994. PMC 372963. PMID 8078435.
- ^ Alvarez I, Wendel JF (December 2003). "Ribosomal ITS sequences and plant phylogenetic inference". Molecular Phylogenetics and Evolution. 29 (3): 417–34. Bibcode:2003MolPE..29..417A. doi:10.1016/S1055-7903(03)00208-2. PMID 14615184.
- ^ Weitemier K, Straub SC, Fishbein M, Liston A (2015). "Intragenomic polymorphisms among high-copy loci: a genus-wide study of nuclear ribosomal DNA in Asclepias (Apocynaceae)". PeerJ. 3: e718. doi:10.7717/peerj.718. PMC 4304868. PMID 25653903.
- ^ a b Päques F, Samson ML, Jordan P, Wegnez M (November 1995). "Structural evolution of the Drosophila 5S ribosomal genes". Journal of Molecular Evolution. 41 (5): 615–21. Bibcode:1995JMolE..41..615P. doi:10.1007/bf00175820. PMID 7490776. S2CID 6911205.
- ^ Sumida M, Kato Y, Kurabayashi A (April 2004). "Sequencing and analysis of the internal transcribed spacers (ITSs) and coding regions in the EcoR I fragment of the ribosomal DNA of the Japanese pond frog Rana nigromaculata". Genes & Genetic Systems. 79 (2): 105–18. doi:10.1266/ggs.79.105. PMID 15215676.
- ^ Nazar RN, Sitz TO, Busch H (February 1976). "Sequence homologies in mammalian 5.8S ribosomal RNA". Biochemistry. 15 (3): 505–8. doi:10.1021/bi00648a008. PMID 1252408.
- ^ Fengyi MY, Jiannong X, Zheming Z (1998). "Sequence differences of rDNA-ITS2 and species-diagnostic PCR assay of Anopheles sinensis and Anopheles anthropophagus from China" (PDF). J Med Coll PLA. 13: 123–128.
- ^ Li, C; Lee, JS; Groebner, JL; Kim, HC; Klein, TA; O'Guinn, ML; Wilkerson, RC (2005). "A newly recognized species in the Anopheles hyrcanus group and molecular identification of related species from the Republic of South Korea (Diptera: Culicidae)" (PDF). Zootaxa. 939: 1–8. doi:10.11646/zootaxa.939.1.1. Archived from the original on October 1, 2012.
- ^ Keil RL, Roeder GS (December 1984). "Cis-acting, recombination-stimulating activity in a fragment of the ribosomal DNA of S. cerevisiae". Cell. 39 (2 Pt 1): 377–86. doi:10.1016/0092-8674(84)90016-3. PMID 6094015.
- ^ Serizawa N, Horiuchi T, Kobayashi T (April 2004). "Transcription-mediated hyper-recombination in HOT1". Genes to Cells. 9 (4): 305–15. doi:10.1111/j.1356-9597.2004.00729.x. PMID 15066122. S2CID 23978914.
- ^ Warmerdam DO, Wolthuis RM (March 2019). "Keeping ribosomal DNA intact: a repeating challenge". Chromosome Research. 27 (1–2): 57–72. doi:10.1007/s10577-018-9594-z. PMC 6394564. PMID 30556094.
