Rhynchocephalia
| Rhynchocephalians | |
|---|---|

| |
| The tuatara (Sphenodon punctatus), the only living rhynchocephalian | |

| |
| Fossil of Vadasaurus, a rhynchocephalian from the Late Jurassic of Germany | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Superorder: | Lepidosauria |
| Order: | Rhynchocephalia Günther 1867 |
| Type species | |
| Sphenodon punctatus Gray, 1842
| |
| Subgroups | |
Rhynchocephalia (/ˌrɪŋkoʊsɪˈfeɪliə/; lit. 'beak-heads') is an order of lizard-like reptiles that includes only one living species, the tuatara (Sphenodon punctatus) of New Zealand. Despite its current lack of diversity, during the Mesozoic rhynchocephalians were a speciose group with high morphological and ecological diversity. The oldest record of the group is dated to the Middle Triassic around 238 to 240 million years ago, and they had achieved global distribution by the Early Jurassic.[1] Most rhynchocephalians belong to the group Sphenodontia ('wedge-teeth'). Their closest living relatives are lizards and snakes in the order Squamata, with the two orders being grouped together in the superorder Lepidosauria.
Once representing the world's dominant group of small reptiles, many of the niches occupied by lizards today were held by rhynchocephalians during the Triassic and Jurassic. Rhynchocephalians underwent a great decline during the Cretaceous, and they had disappeared almost entirely by the beginning of the Cenozoic. While the modern tuatara is primarily insectivorous and carnivorous, the diversity of the group also included the herbivorous eilenodontines, and there were other rhynchocephalians with specialised ecologies like the durophagous sapheosaurs. There were even successful groups of aquatic sphenodontians, such as the pleurosaurs.[2]
History
[edit]Tuatara were originally classified as agamid lizards when they were first described by John Edward Gray in 1831. They remained misclassified until 1867, when Albert Günther of the British Museum noted features similar to birds, turtles, and crocodiles. He proposed the order Rhynchocephalia (from ῥύγχος/rhúnkhos 'beak' and κεφαλή/kephalḗ 'head', meaning "beak head") for the tuatara and its fossil relatives.[3] In 1925, Samuel Wendell Williston proposed the Sphenodontia to include only tuatara and their closest fossil relatives.[4] Sphenodon is derived from Greek σφήν (sphḗn) 'wedge' and ὀδούς (odoús) 'tooth'.[5][6][7] Many disparately related species were subsequently added to the Rhynchocephalia, resulting in what taxonomists call a "wastebasket taxon". These include the superficially similar (both in shape and name) but unrelated rhynchosaurs, which lived in the Triassic.[4] Studies in the 1970s and 1980s demonstrated that rhynchosaurs were unrelated, with computer-based cladistic analysis conducted in the 1980s providing a robust diagnosis for the definition of the group.[8]
Anatomy
[edit]
Rhynchocephalia and their sister group Squamata (which includes lizards, snakes and amphisbaenians) belong to the superorder Lepidosauria, the only surviving taxon within Lepidosauromorpha.
Squamates and rhynchocephalians have a number of shared traits (synapomorphies), including fracture planes within the tail vertebrae allowing caudal autotomy (loss of the tail when threatened), transverse cloacal slits, an opening in the pelvis known as the thyroid fenestra, the presence of extra ossification centres in the limb bone epiphyses, a knee joint where a lateral recess on the femur allows the articulation of the fibula, the development of a sexual segment of the kidney, and a number of traits of the feet bones, including a fused astralago-calcaneun and enlarged fourth distal tarsal, which creates a new joint, along with a hooked fifth metatarsal.[9]
Like some lizards, the tuatara possesses a parietal eye (also called a pineal eye or a third eye) covered by scales at the top of the head formed by the parapineal organ, with an accompanying hole in the skull roof enclosed by the parietal bones, dubbed the "pineal foramen", which is also present in fossil rhynchocephalians. The parietal eye detects light (though it is probably not capable of detecting movement or forming images), monitoring the day-night and seasonal cycles, helping to regulate the circadian rhythm, among other functions.[10][11][12][13][14] While parietal eyes were widespread among early vertebrates, including early reptiles, they have been lost among most living groups.[12]
Rhynchocephalians are distinguished from squamates by a number of traits, including the retention of gastralia (rib-like bones present in the belly of the body, ancestrally present in tetrapods and also present in living crocodilians).[15] Unlike squamates, but similar to the majority of birds, the tuatara lacks a penis. This is a secondary loss, as a penis or squamate-like hemipenes were probably present in the last common ancestor of rhynchocephalians and squamates.[16]

The complete lower temporal bar (caused by the fusion of the jugal and quadtrate/quadratojugal bones of the skull) of the tuatara, often historically asserted to be a primitive feature retained from earlier reptiles, is actually a derived feature among sphenodontians, with primitive lepidosauromorphs and many rhynchocephalians including the most primitive ones having an open lower temporal fenestra without a temporal bar.[17][18] While often lacking a complete temporal bar, the vast majority of rhynchocephalians have a posteriorly directed process (extension) of the jugal bone. All known rhynchocephalians lack the splenial bone present in the lower jaw of more primitive reptiles,[19] with the skulls of all members of Sphenodontia lacking lacrimal bones.[20] The majority of rhynchocephalians also have fused frontal bones of the skull.[21][19] While early rhynchocephalians possessed a tympanic membrane in the ear and a corresponding quadrate conch, similar to those found in lizards, these have been lost in the tuatara and likely other derived rhynchocephalians. This loss may be connected to the development of back and forth motion of the lower jaw.[22]

The dentition of most rhynchocephalians, including the tuatara, is described as acrodont, which is associated with the condition of the teeth being attached to the crest of the jaw bone, lacking tooth replacement and having extensive bone growth fusing the teeth to the jaws resulting in the boundary between the teeth and bone being difficult to discern. This differs from the condition found in most lizards (except acrodontans), which have pleurodont teeth which are attached to the shelf on the inward-facing side of the jaw, and are replaced throughout life. The teeth of the tuatara have no roots, though the teeth of some other rhynchocephalians possess roots.[23] The acrodont dentition appears to be a derived character of rhynchocephalians not found in more primitive lepidosauromorphs.[21] The most primitive rhynchocephalians have either pleurodont teeth or a combination of both pleurodont front and acrodont posterior teeth.[23][19] Some rhynchocephalians differ from these conditions, with Ankylosphenodon having superficially acrodont teeth that continue deeply into the jaw bone, and are fused to the bone at the base of the socket (ankylothecodont).[23] In many derived sphenodontians, the premaxillary teeth at the front of the upper jaw are merged into a large chisel-like structure.[24]
Rhynchocephalians possess palatal dentition (teeth present on the bones of the roof of the mouth). Palatal teeth are ancestrally present in tetrapods, but have been lost in many groups. The earliest rhynchocephalians had teeth present on the palatine, vomer and pterygoid bones, though the vomer and/or the pterygoid teeth are lost in some groups, including the living tuatara, which only has palatine teeth.[25] A distinctive character found in all rhynchocephalians is the enlargement of the tooth row present on the palatine bones. While in other rhynchocephalians the palatine tooth row is oblique to the teeth of the maxilla, in members of Sphenodontinae (including the tuatara) and Eilenodontinae it is orientated parallel to the maxilla. In these groups, during biting, the teeth of the dentary in the lower jaw slot between the maxillary and palatine tooth rows. This arrangement, which is unique among amniotes, permits three point bending of food items,[26] and in combination with propalinal movement (back and forward motion of the lower jaw) allows for a shearing bite.[25][27]

The body size of rhynchocephalians is highly variable. The tuatara has an average total length of 34.8 and 42.7 centimetres (13.7 and 16.8 in) for females and males respectively.[28] Clevosaurus sectumsemper has an estimated total length of 12 centimetres (4.7 in),[29] while large individuals of the largest known terrestrial sphenodontian, Priosphenodon avelasi reached total lengths of just over 100 centimetres (39 in).[30] The aquatic pleurosaurs reached lengths of up to 150 centimetres (59 in).[31]
The tuatara has among the highest known ages of sexual maturity among reptiles,[32] at around 9 to 13 years of age,[33] and has a high longevity in comparison to lizards of similar size,[32] with wild individuals likely reaching 70 years, and possibly over 100 years in age.[34] Such a late onset of sexual maturity and longevity may have or not have been typical of extinct rhynchocephalians.[31][35]
Classification
[edit]
While the grouping of Rhynchocephalia is well supported, the relationships of many taxa to each other are uncertain, varying substantially between studies.[36] In modern cladistics, the clade Sphenodontia includes all rhynchocephalians other than Wirtembergia, as well as Gephyrosaurus and other gephyrosaurids. Gephyrosaurids have been found as more closely related to squamates in some analyses.[37][19] In 2018, two major clades within Sphenodontia were defined, the infraorder Eusphenodontia which is defined by the least inclusive clade containing Polysphenodon, Clevosaurus hudsoni and Sphenodon, which is supported by the presence of three synapomorphies, including the presence of clearly visible wear facets on the teeth of the dentary or maxilla, the premaxillary teeth are merged into a chisel like structure, and the palatine teeth are reduced to a single tooth row, with the presence of an additional isolated tooth. The unranked clade Neosphenodontia is defined as the most inclusive clade containing Sphenodon but not Clevosaurus hudsoni, which is supported by the presence of six synapomorphies, including the increased relative length of the antorbital region of the skull (the part of the skull forward of the eye socket), reaching 1/4 to 1/3 of the total skull length, the posterior (hind) edge of the parietal bone is only slightly curved inward, the parietal foramen is found at the same level or forward of the anterior border of the supratemporal fenestra (an opening of the skull), the palatine teeth are further reduced from the condition in eusphenodontians to a single lateral tooth row, the number of pterygoid tooth rows are reduced to one or none, and the posterior border of the ischium is characterised by a distinctive process.[24] In 2021 the clade Acrosphenodontia was defined, which is less inclusive than Sphenodontia and more inclusive than Eusphenodontia, and includes all sphenodontians with fully acrodont dentition, excluding basal partially acrodont sphenodontians.[38] In 2022 the extinct clade Leptorhynchia was defined, including a variety of neosphenodontians, at least some of which were aquatically adapted, characterised by the elongation of the fourth metacarpal, the presence of a posterior process on the ischium, and the antorbital region of the skulls is between a third and a quarter of the total skull length.[20] The clade Opisthodontia has been used for the grouping of all sphenodontians more closely related to Priosphenodon (a member of Eilenodontinae) than to Sphenodon.[39] Not all studies use this clade, as some studies have found the scope of the clade to be identical to Eilenodontinae.[20]
The family Sphenodontidae has been used to include the tuatara and its closest relatives within Rhynchocephalia. However the grouping has lacked a formal definition, with the included taxa varying substantially between analyses.[37] The closest relatives of the tuatara are placed in the clade Sphenodontinae, which are characterised by a completely closed temporal bar.[18]
The following is a cladogram of Rhynchocephalia after DeMar et al. 2022 (based on maximum parsimony, note that cladogram collapses into a polytomy under Bayesian analysis):[20]
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cladogram after Simoes et al. 2022 (based on Bayesian inference analysis):[18]
| Sphenodontia |
| |||||||||||||||
Clades and genera
[edit]- †Wirtembergia
- †Gephyrosauridae?
- †Bharatagama?
- Sphenodontia Williston 1925
- †Diphydontosaurus
- †Micromenodon
- †Paleollanosaurus?
- †Pelecymala
- †Whitakersaurus
- †Parvosaurus
- Acrosphenodontia Chambi-Trowell et al., 2021
- †Godavarisaurus
- †Planocephalosaurus
- †Theretairus
- †Sphenocondor
- †Rebbanasaurus
- Eusphenodontia Herrera-Flores et al. 2018
- †Opisthiamimus
- †Brachyrhinodon
- †Colobops?
- †Lanceirosphenodon
- †Polysphenodon
- Clevosauridae
- †Sigmala
- †Microsphenodon
- †Trullidens
- Neosphenodontia Herrera-Flores et al. 2018
- †Lamarquesaurus
- †Pamizinsaurus
- †Tingitana
- †Ankylosphenodon
- †Derasmosaurus
- †Opisthodontia
- Sphenodontidae
- †Leptorhynchia DeMar, Jones & Carrano, 2022
-
Skull reconstruction of Gephyrosaurus a likely basal rhynchocephalian
-
Reconstruction of the skull of Diphydontosaurus a basal member of Sphenodontia
-
Reconstruction of the skulls of Clevosaurus hudsoni (A) and Clevosaurus cambrica (B)
-
Skull of the basal eusphenodontian Opisthiamimus
-
Skull of Sphenotitan, an early member of Eilenodontinae
-
Reconstruction of the skull of Navajosphenodon, an early member of Sphenodontinae
-
Reconstruction of the skull of the eilenodontine Priosphenodon
-
Skull of the neosphenodontian Vadasaurus
-
Skull of Pleurosaurus
-
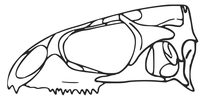
Skull diagram of the tuatara (Sphenodon punctatus)
Ecology
[edit]
The fossil record of rhynchocephalians demonstrates that they were a diverse group that exploited a wide array of ecological niches.[3][26] Early rhynchocephalians possess small ovoid teeth designed for piercing, and were probably insectivores.[40] Like modern tuatara, extinct members of Sphenodontinae were likely generalists with a carnivorous/insectivorous diet.[41] Amongst the most distinct rhynchocephalians are the pleurosaurs, known from the Jurassic of Europe, which were adapted for marine life, with elongated snake-like bodies with reduced limbs, with the specialised Late Jurassic genus Pleurosaurus having an elongated triangular skull highly modified from those of other rhynchocephalians. Pleurosaurs are thought to have been piscivores (consuming fish).[31] Several other lineages of rhynchocephalians have been suggested to have had semi-aquatic habits.[42] Eilenodontines are thought to have been herbivorous, with batteries of wide teeth with thick enamel used to process plant material.[43] The sapheosaurids, such as Oenosaurus and Sapheosaurus from the Late Jurassic of Europe possess broad tooth plates unique amongst tetrapods, and are thought to have been durophagous, with the tooth plates being used to crush hard shelled organisms.[44][37] Sphenovipera from the Jurassic of Mexico has been suggested to have been venomous, based on presence of grooves on two enlarged teeth at the front of the lower jaw[45] though this interpretation has been questioned by other authors.[45] The body of Pamizinsaurus from the Early Cretaceous of Mexico was covered in osteoscutes, similar to those of helodermatid lizards like the Gila monster, which is unique among known sphenodontians, which probably served to protect it against predators.[46]
Evolutionary history
[edit]
The timing of when Rhynchocephalia is estimated to have diverged from Squamata is disputed. Older estimates place the divergence between the Middle Permian and earliest Triassic, around 270 to 252 million years ago,[37] while other authors posit a younger date of around 242 million years ago.[1] The oldest known remains of rhynchocephalians are those of Wirtembergia known from the Erfurt Formation near Vellberg in Southern Germany, dating to the Ladinian stage of the Middle Triassic, around 238-240 million years old, which is also the most primitive rhynchocephalian known.[19] Rhynchocephalians underwent considerable diversification during the Late Triassic,[3] and reached a worldwide distribution across Pangaea by the end of the Triassic, with the Late Triassic-Early Jurassic genus Clevosaurus having 10 species across Asia, Africa, Europe, North and South America.[47] The earliest rhynchocephalians were small animals, but by the Late Triassic the group had evolved a wide range of body sizes.[48] During the Jurassic, rhynchocephalians were the dominant group of small reptiles globally,[49] reaching their apex of morphological diversity during this period, including specialised herbivorous and aquatic forms.[3] The only record of Rhynchocephalians from Asia (excluding the Indian subcontinent, which was not part of Asia during the Mesozoic) are indeterminate remains of Clevosaurus from the Early Jurassic (Sinemurian) aged Lufeng Formation of Yunnan, China. Rhynchocephalians are noticeably absent from younger localities in the region, despite the presence of favourable preservation conditions.[50] Rhynchocephalians remained diverse into the Late Jurassic,[51] and were more abundant than lizards during the Late Jurassic in North America.[49]
Rhynchocephalian diversity declined during the Early Cretaceous, disappearing from North America and Europe after the end of the epoch,[52] and were absent from North Africa[53] and northern South America[54] by the early Late Cretaceous. The cause of the decline of Rhynchocephalia remains unclear, but has often been suggested to be due to competition with advanced lizards and mammals.[55] They appear to have remained prevalent in southern South America during the Late Cretaceous, where lizards remained rare, with their remains outnumbering terrestrial lizards in this region by a factor of 200.[53] Late Cretaceous South American sphenodontians are represented by both Eilenodontinae and Sphenodontidae (including Sphenodontinae).[56] An indeterminate rhynchocephalian is known from a partial lower jaw of a hatchling from the latest Cretaceous or possibly earliest Paleocene Intertrappean Beds, in what was then the isolated landmass of Insular India, which appears to be an acrosphenodontian, possibly related to Godavarisaurus from the Jurassic of India.[51] The youngest undoubted remains of rhynchocephalians outside of New Zealand are those of the sphenodontid Kawasphenodon peligrensis from the early Paleocene (Danian) of Patagonia, shortly after the Cretaceous–Paleogene extinction event.[57] Indeterminate sphenodontine jaw fragments bearing teeth are known from the early Miocene (19–16 million years ago) St Bathans fauna, New Zealand, that are indistinguishable from those of the living tuatara. It is unlikely that the ancestors of the tuatara arrived in New Zealand via oceanic dispersal, and it is thought that they were already present in New Zealand when it separated from Antarctica between 80 and 66 million years ago.[55]
References
[edit]- ^ a b Jones ME, Anderson CL, Hipsley CA, Müller J, Evans SE, Schoch RR (September 2013). "Integration of molecules and new fossils supports a Triassic origin for Lepidosauria (lizards, snakes, and tuatara)". BMC Evolutionary Biology. 13 (1): 208. Bibcode:2013BMCEE..13..208J. doi:10.1186/1471-2148-13-208. PMC 4016551. PMID 24063680.
- ^ Reynoso VH (2000). "An unusual aquatic sphenodontian (Reptilia: Diapsida) from the Tlayua Formation (Albian), central Mexico". Journal of Paleontology. 74 (1): 133–148. Bibcode:2000JPal...74..133R. doi:10.1017/s0022336000031310. S2CID 232346834.
- ^ a b c d Herrera-Flores JA, Stubbs TL, Benton MJ (2017). "Macroevolutionary patterns in Rhynchocephalia: is the tuatara (Sphenodon punctatus) a living fossil?". Palaeontology. 60 (3): 319–328. Bibcode:2017Palgy..60..319H. doi:10.1111/pala.12284. ISSN 1475-4983.
- ^ a b Fraser N, Sues HD, eds. (1994). "Phylogeny" In the Shadow of the Dinosaurs: Early Mesozoic Tetrapods. Cambridge University Press. ISBN 0-521-45242-2.
- ^ Evans SE, Prasad GV, Manhas BK (November 2001). "Rhynchocephalians (Diapsida: Lepidosauria) from the Jurassic Kota Formation of India". Zoological Journal of the Linnean Society. 133 (3): 309–34. doi:10.1111/j.1096-3642.2001.tb00629.x.
- ^ "Sphenodon". Dictionary.com Unabridged (v 1.1). Random House, Inc. Retrieved 8 January 2007.
- ^ Evans SE, Borsuk-Białynicka M (2009). "A small lepidosauromorph reptile from the Early Triassic of Poland" (PDF). Paleontologica Polonica. 65: 179–202.
- ^ Gauthier, J., Estes, R. & De Queiroz, K. (1988). A phylogenetic analysis of Lepidosauromorpha. In Phylogenetic Relationships of the Lizard Families (eds R. Estes and G. Pregill), pp. 15–98. Stanford University Press, Stanford.
- ^ "Rhynchocephalians". University College London. Retrieved 2022-08-09.
- ^ Dendy A (1911). "VI. On the structure, development and morphological interpretation of the pineal organs and adjacent parts of the brain in the tuatara ( sphenodon punctatus )". Philosophical Transactions of the Royal Society B. 201 (274–281): 227–331. doi:10.1098/rstb.1911.0006. ISSN 0264-3960.
- ^ Smith KT, Bhullar BA, Köhler G, Habersetzer J (2 April 2018). "The only known jawed vertebrate with four eyes and the bauplan of the pineal complex". Current Biology. 28 (7): 1101–1107.e2. Bibcode:2018CBio...28E1101S. doi:10.1016/j.cub.2018.02.021. ISSN 0960-9822. PMID 29614279.
- ^ a b Benoit J, Abdala F, Manger P, Rubidge B (2016). "The sixth sense in mammalians forerunners: variability of the parietal foramen and the evolution of the pineal eye in South African Permo-Triassic eutheriodont therapsids". Acta Palaeontologica Polonica. doi:10.4202/app.00219.2015.
- ^ Paulina-Carabajal A, Jiménez-Huidobro P, Triviño LN, Stanley EL, Zaher H, Daza JD (2023), Dozo MT, Paulina-Carabajal A, Macrini TE, Walsh S (eds.), "A Look in to the Neurocranium of Living and Extinct Lepidosauria", Paleoneurology of Amniotes, Cham: Springer International Publishing, pp. 123–177, doi:10.1007/978-3-031-13983-3_5, ISBN 978-3-031-13982-6, retrieved 2023-12-08
- ^ Jones ME, Cree A (December 2012). "Tuatara". Current Biology. 22 (23): R986 – R987. doi:10.1016/j.cub.2012.10.049.
- ^ Vitt LJ, Caldwell JP (2014). "Chapter 20: Rhynchocephalians (Sphenodontids)". Herpetology. Elsevier. pp. 553–554. doi:10.1016/b978-0-12-386919-7.00020-4. ISBN 978-0-12-386919-7.
- ^ Sanger TJ, Gredler ML, Cohn MJ (October 2015). "Resurrecting embryos of the tuatara, Sphenodon punctatus, to resolve vertebrate phallus evolution". Biology Letters. 11 (10): 20150694. doi:10.1098/rsbl.2015.0694. PMC 4650183. PMID 26510679.
- ^ Evans SE, Jones ME (2010). "The Origin, Early History and Diversification of Lepidosauromorph Reptiles". New Aspects of Mesozoic Biodiversity. Lecture Notes in Earth Sciences. Vol. 132. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 27–44. Bibcode:2010LNES..132...27E. doi:10.1007/978-3-642-10311-7_2. ISBN 978-3-642-10310-0.
- ^ a b c Simões TR, Kinney-Broderick G, Pierce SE (March 2022). "An exceptionally preserved Sphenodon-like sphenodontian reveals deep time conservation of the tuatara skeleton and ontogeny". Communications Biology. 5 (1): 195. doi:10.1038/s42003-022-03144-y. PMC 8894340. PMID 35241764.
- ^ a b c d e Sues HD, Schoch RR (2023-11-07). "The oldest known rhynchocephalian reptile from the Middle Triassic (Ladinian) of Germany and its phylogenetic position among Lepidosauromorpha". The Anatomical Record. 307 (4): 776–790. doi:10.1002/ar.25339. ISSN 1932-8486. PMID 37937325.
- ^ a b c d DeMar DG, Jones ME, Carrano MT (2022-12-31). "A nearly complete skeleton of a new eusphenodontian from the Upper Jurassic Morrison Formation, Wyoming, USA, provides insight into the evolution and diversity of Rhynchocephalia (Reptilia: Lepidosauria)". Journal of Systematic Palaeontology. 20 (1): 1–64. doi:10.1080/14772019.2022.2093139. hdl:2440/136608. ISSN 1477-2019. S2CID 252325953.
- ^ a b Ford DP, Evans SE, Choiniere JN, Fernandez V, Benson RB (2021-08-25). "A reassessment of the enigmatic diapsid Paliguana whitei and the early history of Lepidosauromorpha". Proceedings of the Royal Society B: Biological Sciences. 288 (1957): 20211084. doi:10.1098/rspb.2021.1084. ISSN 0962-8452. PMC 8385343. PMID 34428965.
- ^ Evans SE (2016), Clack JA, Fay RR, Popper AN (eds.), "The Lepidosaurian Ear: Variations on a Theme", Evolution of the Vertebrate Ear, Springer Handbook of Auditory Research, vol. 59, Cham: Springer International Publishing, pp. 245–284, doi:10.1007/978-3-319-46661-3_9, ISBN 978-3-319-46659-0, retrieved 2024-01-08
- ^ a b c Jenkins KM, Jones ME, Zikmund T, Boyde A, Daza JD (September 2017). "A Review of Tooth Implantation Among Rhynchocephalians (Lepidosauria)". Journal of Herpetology. 51 (3): 300–306. doi:10.1670/16-146. ISSN 0022-1511. S2CID 90519352.
- ^ a b Herrera-Flores JA, Stubbs TL, Elsler A, Benton MJ (2018-04-06). "Taxonomic reassessment of Clevosaurus latidens Fraser, 1993 (Lepidosauria, Rhynchocephalia) and rhynchocephalian phylogeny based on parsimony and Bayesian inference". Journal of Paleontology. 92 (4): 734–742. Bibcode:2018JPal...92..734H. doi:10.1017/jpa.2017.136. hdl:1983/59126b60-16d8-46d2-b657-954693a39d4e.
- ^ a b Matsumoto R, Evans SE (January 2017). "The palatal dentition of tetrapods and its functional significance". Journal of Anatomy. 230 (1): 47–65. doi:10.1111/joa.12534. PMC 5192890. PMID 27542892.
- ^ a b Jones ME (August 2008). "Skull shape and feeding strategy in Sphenodon and other Rhynchocephalia (Diapsida: Lepidosauria)". Journal of Morphology. 269 (8): 945–966. doi:10.1002/jmor.10634. PMID 18512698.
- ^ Jones ME, O'higgins P, Fagan MJ, Evans SE, Curtis N (July 2012). "Shearing mechanics and the influence of a flexible symphysis during oral food processing in Sphenodon (Lepidosauria: Rhynchocephalia)". Anatomical Record. 295 (7): 1075–1091. doi:10.1002/ar.22487. PMID 22644955.
- ^ Herrel A, Moore JA, Bredeweg EM, Nelson NJ (2010-05-19). "Sexual dimorphism, body size, bite force and male mating success in tuatara: SEXUAL DIMORPHISM IN TUATARA". Biological Journal of the Linnean Society. 100 (2): 287–292. doi:10.1111/j.1095-8312.2010.01433.x.
- ^ Keeble E, Whiteside DI, Benton MJ (April 2018). "The terrestrial fauna of the Late Triassic Pant-y-ffynnon Quarry fissures, South Wales, UK and a new species of Clevosaurus (Lepidosauria: Rhynchocephalia)". Proceedings of the Geologists' Association. 129 (2): 99–119. Bibcode:2018PrGA..129...99K. doi:10.1016/j.pgeola.2017.11.001. hdl:1983/5afdc677-3ea0-4519-813d-6052ef8370ec.
- ^ Apesteguía S, Novas FE (9 October 2003). "Large Cretaceous sphenodontian from Patagonia provides insight into lepidosaur evolution in Gondwana". Nature. 425 (6958): 609–612. Bibcode:2003Natur.425..609A. doi:10.1038/nature01995. PMID 14534584. S2CID 4425130.
- ^ a b c Klein N, Scheyer TM (February 2017). "Microanatomy and life history in Palaeopleurosaurus (Rhynchocephalia: Pleurosauridae) from the Early Jurassic of Germany". Die Naturwissenschaften. 104 (1–2): 4. Bibcode:2017SciNa.104....4K. doi:10.1007/s00114-016-1427-3. PMID 28005148. S2CID 27133670.
- ^ a b Hallmann K, Griebeler EM (June 2018). "An exploration of differences in the scaling of life history traits with body mass within reptiles and between amniotes". Ecology and Evolution. 8 (11): 5480–5494. Bibcode:2018EcoEv...8.5480H. doi:10.1002/ece3.4069. ISSN 2045-7758. PMC 6010814. PMID 29938067.
- ^ Newman DG (July 1988). "Evidence of predation on a young tuatara, Sphenodon punctatus, by kiore, Rattus exulans, on Lady Alice Island". New Zealand Journal of Zoology. 15 (3): 443–446. doi:10.1080/03014223.1988.10422973. ISSN 0301-4223.
- ^ Nelson NJ, Keall SN, Pledger S, Daugherty CH (May 2002). "Male-biased sex ratio in a small tuatara population". Journal of Biogeography. 29 (5–6): 633–640. Bibcode:2002JBiog..29..633N. doi:10.1046/j.1365-2699.2002.00712.x. ISSN 0305-0270.
- ^ Cavasín S, Cerda I, Apesteguia S (2024). "Bone microstructure of Priosphenodon avelasi (Rhynchocephalia: Sphenodontia): paleobiological implications". Acta Palaeontologica Polonica. 69. doi:10.4202/app.01071.2023.
- ^ Romo de Vivar PR, Martinelli AG, Schmaltz Hsiou A, Soares MB (2020-07-02). "A New Rhynchocephalian from the Late Triassic of Southern Brazil Enhances Eusphenodontian Diversity". Journal of Systematic Palaeontology. 18 (13): 1103–1126. Bibcode:2020JSPal..18.1103R. doi:10.1080/14772019.2020.1732488. ISSN 1477-2019. S2CID 216226211.
- ^ a b c d Simões TR, Caldwell MW, Pierce SE (December 2020). "Sphenodontian phylogeny and the impact of model choice in Bayesian morphological clock estimates of divergence times and evolutionary rates". BMC Biology. 18 (1): 191. doi:10.1186/s12915-020-00901-5. PMC 7720557. PMID 33287835.
- ^ Chambi-Trowell SA, Martinelli AG, Whiteside DI, Vivar PR, Soares MB, Schultz CL, et al. (2021-06-03). "The diversity of Triassic South American sphenodontians: a new basal form, clevosaurs, and a revision of rhynchocephalian phylogeny". Journal of Systematic Palaeontology. 19 (11): 787–820. Bibcode:2021JSPal..19..787C. doi:10.1080/14772019.2021.1976292. hdl:1983/af14affc-a26e-426b-83ca-e1833e355882. ISSN 1477-2019. S2CID 240487298.
- ^ Apesteguía S, Novas FE (2003-10-09). "Large Cretaceous sphenodontian from Patagonia provides insight into lepidosaur evolution in Gondwana". Nature. 425 (6958): 609–612. Bibcode:2003Natur.425..609A. doi:10.1038/nature01995. ISSN 0028-0836. PMID 14534584.
- ^ Jones ME (2009). Koppe T, Meyer G, Alt KW, Brook A (eds.). "Dentary Tooth Shape in Sphenodon and Its Fossil Relatives (Diapsida: Lepidosauria: Rhynchocephalia)". Frontiers of Oral Biology. 13. Basel: Karger: 9–15. doi:10.1159/000242382. ISBN 978-3-8055-9229-1. PMID 19828962.
- ^ Villa A, Montie R, Röper M, Rothgaenger M, Rauhut OW (2021-05-03). "Sphenofontis velserae gen. et sp. nov., a new rhynchocephalian from the Late Jurassic of Brunn (Solnhofen Archipelago, southern Germany)". PeerJ. 9: e11363. doi:10.7717/peerj.11363. ISSN 2167-8359. PMC 8101455. PMID 33987027.
- ^ Bever GS, Norell MA (November 2017). "A new rhynchocephalian (Reptilia: Lepidosauria) from the Late Jurassic of Solnhofen (Germany) and the origin of the marine Pleurosauridae". Royal Society Open Science. 4 (11): 170570. doi:10.1098/rsos.170570. PMC 5717629. PMID 29291055.
- ^ Jones ME, Lucas PW, Tucker AS, Watson AP, Sertich JJ, Foster JR, et al. (June 2018). "Neutron scanning reveals unexpected complexity in the enamel thickness of an herbivorous Jurassic reptile". Journal of the Royal Society, Interface. 15 (143): 20180039. doi:10.1098/rsif.2018.0039. PMC 6030635. PMID 29899156.
- ^ Rauhut OW, Heyng AM, López-Arbarello A, Hecker A (2012). Farke AA (ed.). "A new rhynchocephalian from the late jurassic of Germany with a dentition that is unique amongst tetrapods". PLOS ONE. 7 (10): e46839. Bibcode:2012PLoSO...746839R. doi:10.1371/journal.pone.0046839. PMC 3485277. PMID 23118861.
- ^ a b Folinsbee KE, Müller J, Reisz RR (2007-06-12). "Canine grooves: morphology, function, and relevance to venom". Journal of Vertebrate Paleontology. 27 (2): 547–551. doi:10.1671/0272-4634(2007)27[547:CGMFAR]2.0.CO;2. ISSN 0272-4634.
- ^ Reynoso VH (1997-04-16). "A "beaded" sphenodontian (Diapsida: Lepidosauria) from the Early Cretaceous of central Mexico". Journal of Vertebrate Paleontology. 17 (1): 52–59. Bibcode:1997JVPal..17...52R. doi:10.1080/02724634.1997.10010953. ISSN 0272-4634.
- ^ Chambi-Trowell SA, Whiteside DI, Benton MJ, Rayfield EJ (November 2020). Lautenschlager S (ed.). "Biomechanical properties of the jaws of two species of Clevosaurus and a reanalysis of rhynchocephalian dentary morphospace". Palaeontology. 63 (6): 919–939. Bibcode:2020Palgy..63..919C. doi:10.1111/pala.12493. S2CID 220902843.
- ^ Herrera-Flores JA, Elsler A, Stubbs TL, Benton MJ (2021). "Slow and fast evolutionary rates in the history of lepidosaurs". Palaeontology. 65. doi:10.1111/pala.12579. ISSN 1475-4983. S2CID 244019684.
- ^ a b Brownstein CD, Meyer DL, Fabbri M, Bhullar BA, Gauthier JA (2022-11-29). "Evolutionary origins of the prolonged extant squamate radiation". Nature Communications. 13 (1): 7087. Bibcode:2022NatCo..13.7087B. doi:10.1038/s41467-022-34217-5. ISSN 2041-1723. PMC 9708687. PMID 36446761.
- ^ Jones ME (2006). "The Early Jurassic clevosaurs from China (Diapsida: Lepidosauria)". New Mexico Museum of Natural History and Science Bulletin. 37: 548–562.
- ^ a b Anantharaman S, DeMar DG, Sivakumar R, Dassarma DC, Wilson Mantilla GP, Wilson Mantilla JA (2022-06-30). "First rhynchocephalian (Reptilia, Lepidosauria) from the Cretaceous–Paleogene of India". Journal of Vertebrate Paleontology. 42 (1): e2118059. Bibcode:2022JVPal..42E8059A. doi:10.1080/02724634.2022.2118059. ISSN 0272-4634. S2CID 252558728.
- ^ Cleary TJ, Benson RB, Evans SE, Barrett PM (March 2018). "Lepidosaurian diversity in the Mesozoic-Palaeogene: the potential roles of sampling biases and environmental drivers". Royal Society Open Science. 5 (3): 171830. Bibcode:2018RSOS....571830C. doi:10.1098/rsos.171830. PMC 5882712. PMID 29657788.
- ^ a b Apesteguía S, Daza JD, Simões TR, Rage JC (September 2016). "The first iguanian lizard from the Mesozoic of Africa". Royal Society Open Science. 3 (9): 160462. Bibcode:2016RSOS....360462A. doi:10.1098/rsos.160462. PMC 5043327. PMID 27703708.
- ^ Simões TR, Wilner E, Caldwell MW, Weinschütz LC, Kellner AW (August 2015). "A stem acrodontan lizard in the Cretaceous of Brazil revises early lizard evolution in Gondwana". Nature Communications. 6 (1): 8149. Bibcode:2015NatCo...6.8149S. doi:10.1038/ncomms9149. PMC 4560825. PMID 26306778.
- ^ a b Jones ME, Tennyson AJ, Worthy JP, Evans SE, Worthy TH (April 2009). "A sphenodontine (Rhynchocephalia) from the Miocene of New Zealand and palaeobiogeography of the tuatara (Sphenodon)". Proceedings of the Royal Society B: Biological Sciences. 276 (1660): 1385–90. doi:10.1098/rspb.2008.1785. PMC 2660973. PMID 19203920.
- ^ Agnolín FL, Aranciaga Rolando AM, Chimento NR, Novas FE (October 2023). "New small reptile remains from the Late Cretaceous of Patagonia increase morphological diversity of sphenodontids (Lepidosauria)". Proceedings of the Geologists' Association. 135: 36–44. doi:10.1016/j.pgeola.2023.09.007.
- ^ Apesteguía S, Gómez RO, Rougier GW (October 2014). "The youngest South American rhynchocephalian, a survivor of the K/Pg extinction". Proceedings of the Royal Society B: Biological Sciences. 281 (1792): 20140811. doi:10.1098/rspb.2014.0811. PMC 4150314. PMID 25143041.
Further reading
[edit]- Apesteguia S, Rougier GW (2007). "A Late Campanian Sphenodontid Maxilla from Northern Patagonia" (PDF). American Museum Novitates (3581): 1–12. doi:10.1206/0003-0082(2007)3581[1:ALCSMF]2.0.CO;2. Retrieved 2019-03-30.
- Daugherty CH, Cree A, Hay JM, Thompson MB (1990). "Neglected taxonomy and continuing extinctions of tuatara (Sphenodon)". Nature. 347 (6289): 177–179. Bibcode:1990Natur.347..177D. doi:10.1038/347177a0. S2CID 4342765.
- Evans SE (November 2003). "At the feet of the dinosaurs: the early history and radiation of lizards" (PDF). Biological Reviews of the Cambridge Philosophical Society. 78 (4): 513–51. doi:10.1017/S1464793103006134. PMID 14700390. S2CID 4845536.
- Gemmell NJ, Rutherford K, Prost S, Tollis M, Winter D, Macey JR, et al. (August 2020). "The tuatara genome reveals ancient features of amniote evolution". Nature. 584 (7821): 403–409. doi:10.1038/s41586-020-2561-9. PMC 7116210. PMID 32760000.
External links
[edit] Media related to Sphenodontia at Wikimedia Commons
Media related to Sphenodontia at Wikimedia Commons Data related to Rhynchocephalia at Wikispecies
Data related to Rhynchocephalia at Wikispecies
- Rhynchocephalia
- Taxa named by Albert Günther
- Tetrapod orders
- Extant Middle Triassic first appearances
- Ladinian first appearances
- Middle Triassic taxonomic orders
- Late Triassic taxonomic orders
- Early Jurassic taxonomic orders
- Middle Jurassic taxonomic orders
- Late Jurassic taxonomic orders
- Early Cretaceous taxonomic orders
- Late Cretaceous taxonomic orders
- Paleocene taxonomic orders
- Eocene taxonomic orders
- Oligocene taxonomic orders
- Miocene taxonomic orders
- Pliocene taxonomic orders
- Pleistocene taxonomic orders
- Holocene taxonomic orders