Phosphazene
Phosphazenes refer to various classes of organophosphorus compounds featuring phosphorus(V) with a double bond between P and N. One class of phosphazenes have the formula R−N=P(−NR2)3. These phosphazenes are also known as iminophosphoranes and phosphine imides. They are superbases.[1]
BEMP and P4-t-Bu|t-Bu-P4
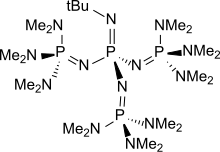
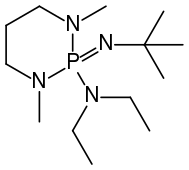
[edit]Well known phosphazene bases are BEMP (2-tert-Butylimino-2-diEthylamino-1,3-diMethylperhydro-1,3,2-diazaPhosphorine) with an acetonitrile pKa of the conjugate acid of 27.6 and the phosphorimidic triamide t-Bu-P4 (pKBH+ = 42.7) also known as Schwesinger base.[2] BEMP and P4-t-Bu|t-Bu-P4 have attracted attention because they are low-nucleophilic, which precludes their participating in competing reactions. Being non-ionic ("charge-neutral"), they are soluble in nonpolar solvents. Protonation takes place at a doubly bonded nitrogen atom.[3] The pKa's of tert-Bu−(H)N=P(−N=P(−NR2)3)3]+, where R = Me and pyrrolidinyl, are 42.7 and 44, respectively. These are the highest pKa recorded for the conjugate acid of charge-neutral molecular base.[4]
-
t-Bu-P4
-
BEMP
In one implemention, t-Bu-P4 catalyzes the conversion of pivaldehyde to the alcohol:[5] Phosphazene bases have been used as basic titrants in non-aqueous acid–base titrations.
Other classes of phosphazenes
[edit]Also called phosphazenes are represented with the formula (−N=P(−X)2−)n, where X = halogen, alkoxy group, amide and other organyl groups. One example is hexachlorocyclotriphosphazene (−N=P(−Cl)2−)3. Bis(triphenylphosphine)iminium chloride [Ph3P=N=PPh3]+Cl−is also referred to as a phosphazene, where Ph = phenyl group. The present article focuses on those phosphazenes with the formula R−N=P(−NR2)3.
See also
[edit]- Verkade bases feature P(III) with three amido substituents and a transannular amine
- Cyclodiphosphazane
- Hexachlorophosphazene
- Polyphosphazene
References
[edit]- ^ Superbases for Organic Synthesis: Guanidines, Amidines, Phosphazenes and Related Organocatalysts Tsutomu Ishikawa ISBN 978-0-470-51800-7
- ^ Schwesinger, Reinhard; Schlemper, Helmut (1987). "Peralkylated Polyaminophosphazenes— Extremely Strong, Neutral Nitrogen Bases". Angewandte Chemie International Edition in English. 26 (11): 1167. doi:10.1002/anie.198711671.
- ^ Schwesinger, Reinhard; Hasenfratz, Christian; Schlemper, Helmut; Walz, Leonhard; Peters, Eva-Maria; Peters, Karl; von Schnering, Hans Georg (1993). "How Strong and How Hindered Can Uncharged Phosphazene Bases Be?". Angewandte Chemie International Edition in English. 32 (9): 1361–1363. doi:10.1002/anie.199313611.
- ^ Saame, Jaan; Rodima, Toomas; Tshepelevitsh, Sofja; Kütt, Agnes; Kaljurand, Ivari; Haljasorg, Tõiv; Koppel, Ilmar A.; Leito, Ivo (2016). "Experimental Basicities of Superbasic Phosphonium Ylides and Phosphazenes". The Journal of Organic Chemistry. 81 (17): 7349–7361. doi:10.1021/acs.joc.6b00872. PMID 27392255.
- ^ Suzawa, Koichi; Ueno, Masahiro; Wheatley, Andrew E. H.; Kondo, Yoshinori (2006). "Phosphazene base-promoted functionalization of aryltrimethylsilanes". Chemical Communications (46): 4850–4852. doi:10.1039/b611090h. PMID 17345750.