Phenylpropanoids metabolism
The biosynthesis of phenylpropanoids involves a number of enzymes.
From amino acids to cinnamates
[edit]In plants, all phenylpropanoids are derived from the amino acids phenylalanine and tyrosine.
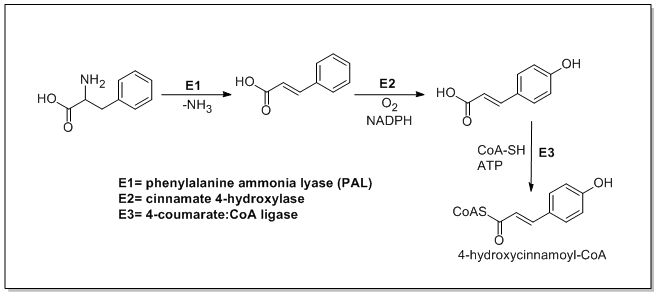
Phenylalanine ammonia-lyase (PAL, a.k.a. phenylalanine/tyrosine ammonia-lyase) is an enzyme that transforms L-phenylalanine and tyrosine into trans-cinnamic acid and p-coumaric acid, respectively.
Trans-cinnamate 4-monooxygenase (cinnamate 4-hydroxylase) is the enzyme that transforms trans-cinnamate into 4-hydroxycinnamate (p-coumaric acid). 4-Coumarate-CoA ligase is the enzyme that transforms 4-coumarate (p-coumaric acid) into 4-coumaroyl-CoA.[1]
Enzymes associated with biosynthesis of hydroxycinnamic acids
[edit]- Cinnamyl-alcohol dehydrogenase (CAD), an enzyme that transforms cinnamyl alcohol into cinnamaldehyde
- Sinapine esterase, an enzyme that transforms sinapoylcholine into sinapate (sinapic acid) and choline
- Trans-cinnamate 2-monooxygenase, an enzyme that transforms trans-cinnamate (cinnamic acid) into 2-hydroxycinnamate
- Caffeate O-methyltransferase, an enzyme that transforms caffeic acid into ferulic acid
- Caffeoyl-CoA O-methyltransferase, an enzyme that transforms caffeoyl-CoA into feruloyl-CoA
- 5-O-(4-coumaroyl)-D-quinate 3'-monooxygenase, an enzyme that transforms trans-5-O-(4-coumaroyl)-D-quinate into trans-5-O-caffeoyl-D-quinate
- Sinapoylglucose—choline O-sinapoyltransferase, an enzyme that transforms 1-O-sinapoyl-beta-D-glucose into sinapoylcholine (sinapine)
- Sinapoylglucose—malate O-sinapoyltransferase, an enzyme that transforms 1-O-sinapoyl-beta-D-glucose into sinapoyl-(S)-malate
- Cinnamoyl-CoA reductase, an enzyme that transforms cinnamoyl-CoA from cinnamaldehyde
Conjugation enzymes
[edit]These enzymes conjugate phenylpropanoids to other molecules.
- 2-coumarate O-beta-glucosyltransferase, the enzyme that transforms trans-2-hydroxycinnamate into trans-beta-D-glucosyl-2-hydroxycinnamate
- Hydroxycinnamate 4-beta-glucosyltransferase, the enzyme that transforms p-coumaric acid into 4-O-beta-D-glucosyl-4-hydroxycinnamate
- Shikimate O-hydroxycinnamoyltransferase, the enzyme that transforms 4-coumaroyl-CoA into 4-coumaroylshikimate
- Quinate O-hydroxycinnamoyltransferase, the enzyme that transforms feruloyl-CoA into O-feruloylquinate
- Sinapate 1-glucosyltransferase, the enzyme that transforms sinapate (sinapic acid) into 1-sinapoyl-D-glucose
- Coniferyl-alcohol glucosyltransferase, the enzyme that transforms coniferyl alcohol into coniferin
Deconjugation enzymes
[edit]- Coniferin beta-glucosidase, in the glucosidase that transforms coniferin into coniferol
Stilbenoids biosynthesis
[edit]- Pinosylvin synthase, an enzyme that transforms pinosylvin from cinnamoyl-CoA
- Trihydroxystilbene synthase, an enzyme that transforms 4-coumaroyl-CoA to resveratrol.
An alternative bacterial ketosynthase-directed stilbenoids biosynthesis pathway exists in Photorhabdus bacterial symbionts of Heterorhabditis nematodes, producing 3,5-dihydroxy-4-isopropyl-trans-stilbene for antibiotic purposes.[2]
- Scopoletin glucosyltransferase, the enzyme that transforms scopoletin into scopolin
Chalcones biosynthesis
[edit]4-Coumaroyl-CoA can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalconoids, which contain two phenyl rings. Naringenin-chalcone synthase is an enzyme that catalyzes the following conversion:
- 3-malonyl-CoA + 4-coumaroyl-CoA → 4 CoA + naringenin chalcone + 3 CO2
Flavonoids biosynthesis
[edit]Conjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone.
Biodegradation
[edit]Hydroxycinnamic acids degradation
[edit]- Caffeate 3,4-dioxygenase is an enzyme that uses 3,4-dihydroxy-trans-cinnamate (caffeic acid) and oxygen to produce 3-(2-carboxyethenyl)-cis,cis-muconate.
References
[edit]- ^ Ververidis Filippos, F.; Trantas Emmanouil; Douglas Carl; Vollmer Guenter; Kretzschmar Georg; Panopoulos Nickolas (October 2007). "Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health". Biotechnology Journal. 2 (10): 1214–34. doi:10.1002/biot.200700084. PMID 17935117.
- ^ Joyce SA, Brachmann AO, Glazer I, Lango L, Schwär G, Clarke DJ, Bode HB (2008). "Bacterial biosynthesis of a multipotent stilbene". Angew Chem Int Ed Engl. 47 (10): 1942–5. CiteSeerX 10.1.1.603.247. doi:10.1002/anie.200705148. PMID 18236486.