Pertuzumab
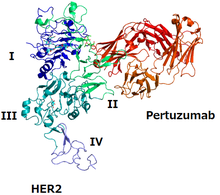 The structure of HER2 and pertuzumab | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Target | HER2 |
| Clinical data | |
| Trade names | Perjeta |
| Other names | 2C4 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| | |
Pertuzumab, sold under the brand name Perjeta, is a monoclonal antibody used in combination with trastuzumab and docetaxel for the treatment of metastatic HER2-positive breast cancer; it also used in the same combination as a neoadjuvant in early HER2-positive breast cancer.[4]
Side effects in more than half the people taking it include diarrhea, hair loss, and loss of neutrophils; more than 10% experience loss of red blood cells, hypersensitivity or allergic reaction, infusion reactions, decreased appetite, insomnia, distortions in the sense of taste, inflammation of the mouth or lips, constipation, rashes, nail disease, and muscle pain.[3] Women who are pregnant or planning on getting pregnant should not take it, it was not studied in people with certain heart conditions and should be used in caution in such people, and it should not be used with an anthracycline.[3] It is unknown if pertuzumab interacts with doxorubicin.[3]
It is the first-in-class of a kind of drug called a "HER dimerization inhibitor" — it inhibits the dimerization of HER2 with other HER receptors, which prevents them from signalling in ways that promote cell growth and proliferation.[6]
It was discovered and developed by Genentech and was first approved in 2012.[7][8]
Medical uses
[edit]Pertuzumab is administered as an intravenous infusion in combination with trastuzumab and docetaxel as a first line treatment for HER2-positive metastatic breast cancer.[4][3] It is also used in the same combination as a neoadjuvant (given to reduce the size of a tumor, prior to surgery or radiation) for HER2-positive early breast cancer; as of 2016 this use had not been shown to increase survival.[4]
As of 2016[update], pertuzumab has not been studied in people with a left ventricular ejection fraction value of ≤ 50% normal, a prior history of congestive heart failure, or conditions that could impair left ventricular function like uncontrolled hypertension, recent heart attacks, or serious cardiac arrhythmia.[3] Caution should be used combining pertuzumab with an anthracycline.[3] There is also no safety data available for use of pertuzumab in combination with doxorubicin.[3]
Women of child-bearing age should use contraception while taking pertuzumab; it may damage the fetus in pregnant women, and it may be secreted in breast milk.[3]
Adverse effects
[edit]In clinical trials of the three-agent combination therapy in metastatic breast cancer, adverse effects occurring in more than half the people taking it included diarrhea, hair loss, and loss of neutrophils; more than 10% of people experienced loss of neutrophils with fever, and loss of leukocytes.[3] After docetaxel was dropped in some people, the most common adverse effects were diarrhea (28.1%), upper respiratory tract infection (18.3%), rash (18.3%), headache (17.0%), fatigue (13.4%), swelling of nasal passages and throat (often due to catching the common cold) (17.0%), weakness (13.4%), itchiness (13.7%), joint pain (11.4%), nausea (12.7%), pain in an extremity (13.4%), back pain (12.1%) and cough (12.1%).[3]
In clinical trials of the neoadjuvant use of the combination, more than 50% of people had hair loss and loss of neutrophils.[3]
In both uses, more than 10% of people additionally experienced: loss of red blood cells, hypersensitivity or allergic reaction, infusion reactions, decreased appetite, insomnia, distortions in the sense of taste, inflammation of the mouth or lips, constipation, rashes, nail disease, and muscle pain.[3]
Pharmacology
[edit]The metabolism of pertuzumab has not been directly studied; in general antibodies are cleared principally by catabolism. The median clearance of pertuzumab was 0.235 liters/day and the median half-life was 18 days.[3]
Mechanism of action
[edit]HER2 is an extracellular receptor—a receptor tyrosine kinase - that when activated, sets off signal transduction through several pathways that stimulate cell proliferation and cell growth; if overexpressed it can cause uncontrollable growth. HER2 positive breast cancer is caused by ERBB2 gene amplification that results in overexpression of HER2 in approximately 15-30% of breast cancer tumors.[9]
Like many receptors, HER2 normally combines another protein in order to function (a process called dimerization); it can bind with a second HER2 receptor (acting as a homodimer) and it can heterodimerize with a different receptor of the HER family. The most potent dimer for activating signalling pathways is HER2/HER3.[6]
The epitope for pertuzumab is the domain of HER2 where it binds to HER3, and pertuzumab prevents the HER2/HER3 dimer from forming, which blocks signalling by the dimer.[6][10] Trastuzumab is another monoclonal antibody against HER2; its epitope is the domain where HER2 binds to another HER2 protein.[6] The two mAbs together prevent HER2 from functioning.[6]
Chemistry and manufacturing
[edit]Pertuzumab is an immunoglobulin G1 with a variable region against the human HER2 protein, a human-mouse monoclonal 2C4 heavy chain, disulfide bound with a human-mouse monoclonal 2C4 κ-chain.[11]
It is manufactured recombinantly in CHO cells.[4]
History
[edit]The monoclonal antibody 2C4 appears to have first been published in 1990 by scientists from Genentech,[12] the same year that F. Hoffmann-La Roche AG acquired a majority stake in Genentech.[13]
By 2003, Genentech understood that 2C4 prevented HER2 dimerizing with other HER receptors and had begun Phase I trials, aiming for a broad range of cancers, not just ones overexpressing HER2. It was the first known HER dimerization inhibitor.[14]
In 2005, Genentech presented poor results of Phase II trials of pertuzumab as a single agent in prostate, breast, and ovarian cancers, and said that it intended to continue developing it in combination with other drugs for ovarian cancer.[15][16]
In 2007, Genentech dropped the trade name Omnitarg.[17][18]
In March 2009, Roche acquired Genentech.[19][20]
In 2012, the results were published of the CLEOPATRA trial, a randomized placebo-controlled Phase III trial of pertuzumab in combination with trastuzumab and docetaxel in HER2-positive metastatic breast cancer.[21] Pertuzumab received US FDA approval for the treatment of HER2-positive metastatic breast cancer later that year.[8] Results of a Phase II trial in the neoadjuvant setting, NeoSphere, published in 2012,[22] and results of a Phase II cardiac safety study in the same population, Tryphaena, published in 2013.[23] The FDA approved the neoadjuvant indication in 2013.[24]
Pertuzumab was approved for medical use in the European Union in 2013.[3][5]
Society and culture
[edit]Economics
[edit]As of 2016[update], in the US each cycle of the three-drug combination given every three weeks costs around US$8,500, not including ancillary care costs.[25]
In the UK, a NICE evaluation in 2015, made a preliminary finding that the drug combination was not cost effective, and NICE rejected the drug in the neoadjuvant setting in May 2016, primarily because it was unknown if the drug combination provided a survival benefit.[26][27][28] This decision was subsequently reversed six months later and pertuzumab became the first new breast cancer drug to be approved by NICE for routine NHS funding in almost a decade after Roche pledged to provide the drug to the NHS at an undisclosed discount for patients in the neoadjuvant setting and to share the long–term financial risks.[29]
References
[edit]- ^ a b "AUSTRALIAN PRODUCT INFORMATION – Perjeta® (pertuzumab)". Retrieved 12 July 2024.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ a b c d e f g h i j k l m n o "Perjeta 420 mg Concentrate for Solution for Infusion — Summary of Product Characteristics (SmPC)". (emc). 2 July 2021. Archived from the original on 3 January 2022. Retrieved 3 January 2022.
- ^ a b c d e "Perjeta- pertuzumab injection, solution, concentrate". DailyMed. Archived from the original on 25 March 2021. Retrieved 3 January 2022.
- ^ a b "Perjeta EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 16 September 2021. Retrieved 3 January 2022.
- ^ a b c d e Harbeck N, Beckmann MW, Rody A, Schneeweiss A, Müller V, Fehm T, et al. (March 2013). "HER2 Dimerization Inhibitor Pertuzumab — Mode of Action and Clinical Data in Breast Cancer". Breast Care. 8 (1): 49–55. doi:10.1159/000346837. PMC 3971793. PMID 24715843.
- ^ "Drug Approval Package: Perjeta (pertuzumab) Injection NDA #125409". U.S. Food and Drug Administration (FDA). 3 August 2012. Archived from the original on 3 January 2022. Retrieved 3 January 2022.
- ^ a b "FDA approves Perjeta for type of late-stage breast cancer". U.S. Food and Drug Administration (FDA). 8 June 2012. Archived from the original on 1 November 2012. Retrieved 3 January 2022.
- ^ Mitri Z, Constantine T, O'Regan R (2012). "The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy". Chemotherapy Research and Practice. 2012: 743193. doi:10.1155/2012/743193. PMC 3539433. PMID 23320171.
- ^ Badache A, Hynes NE (April 2004). "A new therapeutic antibody masks ErbB2 to its partners". Cancer Cell. 5 (4): 299–301. doi:10.1016/s1535-6108(04)00088-1. PMID 15093533.
- ^ "Proposed INN: List 89" (PDF). WHO Drug Information. 17 (3). 2003. Archived (PDF) from the original on 9 August 2020. Retrieved 5 October 2020.
- ^ Fendly BM, Winget M, Hudziak RM, Lipari MT, Napier MA, Ullrich A (March 1990). "Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product" (PDF). Cancer Research. 50 (5): 1550–1558. PMID 1689212. Archived (PDF) from the original on 29 September 2019. Retrieved 2 November 2016., referenced in Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J (June 2001). "Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells" (PDF). Cancer Research. 61 (12): 4744–4749. PMID 11406546. Archived (PDF) from the original on 29 September 2019. Retrieved 2 November 2016.
- ^ Fisher LM (1 October 2000). "Genentech: Survivor Strutting Its Stuff". The New York Times. Archived from the original on 20 December 2016. Retrieved 19 February 2017.
- ^ Albanell J, Codony J, Rovira A, Mellado B, Gascón P (2003). "Mechanism of Action of Anti-Her2 Monoclonal Antibodies: Scientific Update on Trastuzumab and 2c4". New Trends in Cancer for the 21stCentury. Advances in Experimental Medicine and Biology. Vol. 532. Springer. pp. 253–68. doi:10.1007/978-1-4615-0081-0_21. ISBN 978-0-306-47762-1. PMID 12908564.
- ^ "Press Release: Data From Omnitarg Clinical Program Presented at American Society of Clinical Oncology Meeting". Genentech. 15 May 2005. Archived from the original on 6 April 2017. Retrieved 2 November 2016.
- ^ "Genentech's Omnitarg fails in Phase II". Pharma Times. 16 May 2005. Archived from the original on 24 February 2021. Retrieved 2 November 2016.
- ^ "Correction: Letter from the Editor". Cancer Oncology News: 3. February 2012. Archived from the original on 4 November 2016. Retrieved 2 November 2016.
- ^ "Press release: Roche in the first half of 2007". Roche. 19 July 2007. Archived from the original on 4 November 2016. Retrieved 2 November 2016.
- ^ Morse A (10 May 2006). "Chugai Shares Post Healthy Gain On Prospects for Cancer Drug". The Wall Street Journal. Archived from the original on 20 October 2021. Retrieved 26 September 2008.
- ^ "Roche Makes $43.7B Bid for Genentech". Genetic Engineering & Biotechnology News. 21 July 2008. Archived from the original on 3 February 2009. Retrieved 26 September 2008.
- ^ Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, et al. (January 2012). "Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer". The New England Journal of Medicine. 366 (2): 109–119. doi:10.1056/nejmoa1113216. PMC 5705202. PMID 22149875.
- ^ Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. (January 2012). "Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial". The Lancet. Oncology. 13 (1): 25–32. doi:10.1016/s1470-2045(11)70336-9. PMID 22153890. cited in Mates M, Fletcher GG, Freedman OC, Eisen A, Gandhi S, Trudeau ME, Dent SF (March 2015). "Systemic targeted therapy for her2-positive early female breast cancer: a systematic review of the evidence for the 2014 Cancer Care Ontario systemic therapy guideline". Current Oncology. 22 (Suppl 1): S114–S122. doi:10.3747/co.22.2322. PMC 4381787. PMID 25848335.
- ^ Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. (September 2013). "Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA)". Annals of Oncology. 24 (9): 2278–2284. doi:10.1093/annonc/mdt182. PMID 23704196.
- ^ "FDA approves Perjeta for neoadjuvant breast cancer treatment". U.S. Food and Drug Administration (FDA) (Press release). 30 September 2013. Archived from the original on 10 October 2013. Retrieved 3 January 2022.
- ^ Durkee BY, Qian Y, Pollom EL, King MT, Dudley SA, Shaffer JL, et al. (March 2016). "Cost-Effectiveness of Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer". Journal of Clinical Oncology. 34 (9): 902–909. doi:10.1200/jco.2015.62.9105. PMC 5070553. PMID 26351332.
- ^ Fleeman N, Bagust A, Beale S, Dwan K, Dickson R, Proudlove C, Dundar Y (January 2015). "Pertuzumab in combination with trastuzumab and docetaxel for the treatment of HER2-positive metastatic or locally recurrent unresectable breast cancer". PharmacoEconomics. 33 (1): 13–23. doi:10.1007/s40273-014-0206-2. PMID 25138171. S2CID 8470253.
- ^ "Breast cancer (HER2 positive, metastatic) - pertuzumab (with trastuzumab and docetaxel) [ID523]". NICE. 1 September 2016. Archived from the original on 4 November 2016. Retrieved 2 November 2016.
- ^ McKee S (20 May 2016). "NICE rejects Roche's breast cancer drug Perjeta". Pharma Times. Archived from the original on 3 March 2021. Retrieved 2 November 2016.
- ^ Yip A (22 November 2016). "NICE U-Turns and Backs Approval of Roche's Perjeta for HER2-Positive Breast Cancer". Pharmalive. Archived from the original on 7 April 2017. Retrieved 7 April 2017.
Further reading
[edit]- Dean L (2015). "Pertuzumab Therapy and ERBB2 (HER2) Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520364. Bookshelf ID: NBK315949.
