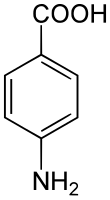
4-Aminobenzoic acid
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Aminobenzoic acid | |||
| Other names
para-Aminobenzoic acid
p-Aminobenzoic acid PABA Vitamin B10 Vitamin Bx Bacterial vitamin H1 | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.231 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C7H7NO2 | |||
| Molar mass | 137.138 g·mol−1 | ||
| Appearance | White-grey crystals | ||
| Density | 1.374 g/mL | ||
| Melting point | 187 to 189 °C (369 to 372 °F; 460 to 462 K) | ||
| Boiling point | 340 °C (644 °F; 613 K) | ||
| 1 g/170 mL (25 °C) 1 g/90 mL (90 °C) | |||
| Acidity (pKa) | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
eye irritant, some persons may be allergic to this compound | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
4-Aminobenzoic acid (also known as para-aminobenzoic acid or PABA because the two functional groups are attached to the benzene ring across from one another in the para position) is an organic compound with the formula H2NC6H4CO2H. PABA is a white crystalline solid,[3] although commercial samples can appear gray. It is slightly soluble in water. It consists of a benzene ring substituted with amino and carboxyl groups. The compound occurs extensively in the natural world.
Production and occurrence
[edit]In industry, PABA is prepared mainly by two routes:
- Reduction of 4-nitrobenzoic acid
- Hoffman degradation of the monoamide derived from terephthalic acid.[4]
Food sources of PABA include liver, brewer's yeast (and unfiltered beer), kidney, molasses, mushrooms, and whole grains.[5] Other food sources of PABA include spinach, liver, and oat seeds.[6]
Biology
[edit]Biochemistry
[edit]
PABA is an intermediate in the synthesis of folate by bacteria, plants, and fungi.[7] Many bacteria, including those found in the human intestinal tract such as E. coli, generate PABA from chorismate by the combined action of the enzymes 4-amino-4-deoxychorismate synthase and 4-amino-4-deoxychorismate lyase.[8] Plants produce PABA in their chloroplasts, and store it as a glucose ester (pABA-Glc) in their tissues. Humans lack the enzymes to convert PABA to folate and so require folate from dietary sources, such as green leafy vegetables. In humans, PABA is considered nonessential and, although it has been referred to historically as "vitamin Bx", is no longer recognized as a vitamin[7] because the typical human gut microbiome generates PABA on its own.
Sulfonamide drugs are structurally similar to PABA, and their antibacterial activity is due to their ability to interfere with the conversion of PABA to folate by the enzyme dihydropteroate synthetase. Thus, bacterial growth is limited through folate deficiency.[9]
Medical use
[edit]The potassium salt is used as a drug against fibrotic skin disorders, such as Peyronie's disease, under the brand name Potaba.[10] PABA is also occasionally used in pill form by sufferers of irritable bowel syndrome to treat its associated gastrointestinal symptoms, and in nutritional epidemiological studies to assess the completeness of 24-hour urine collection for the determination of urinary sodium, potassium, or nitrogen levels. PABA derivatives have also been proposed to function as acetylcholinesterase inhibitors in diseases that cause deficient cholinergic systems, such as Alzheimer's Disease.[11]
Nutritional supplement
[edit]Despite the lack of any recognized syndromes of PABA deficiency in humans, except for those who lack the colonic bacteria that generate PABA, many claims of benefit are made by commercial suppliers of PABA as a nutritional supplement. The benefit is claimed for fatigue, irritability, depression, weeping eczema (moist eczema), scleroderma (premature hardening of the skin), patchy pigment loss in the skin (vitiligo), and premature grey hair.[12]
Commercial and industrial use
[edit]PABA finds use in the biomedical sector. Its derivatives are found as a structural component in 1.5% of a database of 12111 commercial drugs.[13] Other uses include its conversion to specialty azo dyes and crosslinking agents. PABA is also used as a biodegradable pesticide, though its use is now limited due to evolution of new variants of bio-pesticides. Specifically, studies have shown that PABA photodegrades through an O2-mediated pathway in which PABA is oxidized by O2 via hydrogen abstraction and decarboxylation.[14]
In the past, PABA was widely used in sunscreens as a UV filter. It is a UVB absorber, meaning it can absorb wavelengths between 290 and 320 nm.[15] while still allowing UVA wavelengths between 320-400 nm to pass through, producing a tan.[16] The chemical structure of PABA, with the amino and carboxyl groups being para to each other, allows for easy electron delocalization, which reduces the gap between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). This makes it easier for the electrons in PABA to transition to a higher energy state upon absorbing light. Patented in 1943, PABA was one of the first active ingredients to be used in sunscreen.[17] The first in vivo studies on mice showed that PABA reduced UV damage. In addition, it was shown to protect against skin tumors in rodents, as shown by a 1975 study ran by Dr. Diane Sekura Snyder and Dr. Marian May.[18] However, animal and in vitro studies in the early 1980s suggested PABA might increase the risk of cellular UV damage.[19] On the basis of these studies, as well as problems with allergies and clothing discoloration, PABA fell out of favor as a sunscreen. In 2008 it was banned as a sunscreen ingredient in the European Union and in 2019 the FDA proposed its limited use.[20] However, water-insoluble PABA derivatives such as padimate O are currently used in some cosmetic products including mascara, concealer, and matte lipsticks.[21]
As of 2008, the advancement of new sunscreen is focused on developing a broad spectrum of active ingredients that provide consistent protection across all wavelengths, including UVA. Researchers are considering the PABA–TiO2 Hybrid Nanostructures that result from the method of aqueous in situ synthesis with PABA and TiO2.[16]
Safety considerations
[edit]PABA is largely nontoxic; the median lethal dose of PABA in dogs (oral) is 2 g/kg.[4] Allergic reactions, specifically Allergic Contact Dermatitis and Photocontact Dermatitis,[22] to PABA can occur. It is formed in the metabolism of certain ester local anesthetics, and many allergic reactions to local anesthetics are the result of reactions to PABA.[23]
References
[edit]- ^ van de Graaf, Bas (1981). "Substituent effects. 7. Microscopic dissociation constants of 4-amino- and 4-(dimethylamino)benzoic acid". J. Org. Chem. 46 (4): 653–657. doi:10.1021/jo00317a002.
- ^ Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. pp. 5–89. ISBN 978-1-4987-5428-6.
- ^ PubChem. "4-Aminobenzoic Acid". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-11-19.
- ^ a b Maki, T.; Takeda, K. (2000). "Benzoic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a03_555. ISBN 3-527-30673-0.
- ^ "Nutritional Health Resource". Archived from the original on 2009-12-16. Retrieved 2009-11-21.
- ^ Henry RJ (1943). "The Mode of Action of Sulfonamides". American Society for Microbiology. 7 (4): 175–245. doi:10.1128/br.7.4.175-262.1943. PMC 440870. PMID 16350088.
- ^ a b "Para-aminobenzoic acid". Medline Plus Medical Encyclopedia. United States National Institutes of Health. Retrieved 24 January 2014.
- ^ Folate Synthesis (Abstract)
- ^ Brown GM (1962). "The biosynthesis of folic acid. II. Inhibition by sulfonamides". J. Biol. Chem. 237 (2): 536–40. doi:10.1016/S0021-9258(18)93957-8. PMID 13873645.
- ^ "Compound Summary on PubChem". PubChem. National Institute of Health: National Library of Medicine. 2006. Retrieved 2006-04-05.
- ^ Correa-Basurto J (2005). "p-Aminobenzoic acid derivatives as acetylcholinesterase inhibitors". Eur. J. Med. Chem. 40 (7): 732–5. doi:10.1016/j.ejmech.2005.03.011. PMID 15935907.
- ^ "Health Library (Supplements) PABA". Archived from the original on 2017-08-04. Retrieved 2017-08-04.
- ^ Kluczyk, Alicja; Popek, Tomasz; Kiyota, Taira; de Macedo, Pierre; Stefanowicz, Piotr; Lazar, Carmen; Konishi, Yasuo (2002). "Drug Evolution: p-Aminobenzoic Acid as a Building Block". Current Medicinal Chemistry. 9 (21): 1871–1892. doi:10.2174/0929867023368872. ISSN 0929-8673. PMID 12369873.
- ^ Zhang S (2016). "Unveiling self-sensitized photodegradation pathways by DFT calculations: A case of sunscreen p-aminobenzoic acid". Chemosphere. 163: 227–33. Bibcode:2016Chmsp.163..227Z. doi:10.1016/j.chemosphere.2016.08.028. PMID 27529387.
- ^ Melanoma Madness The scientific flap over sunscreens and skin cancer -- Chemical studies, Science News Online, 6/6/98 (accessed 10/1/2009, 2009)
- ^ a b Rahal, R.; Daniele, S.; Hubert-Pfalzgraf, L. G.; Guyot-Ferréol, V.; Tranchant, J (2008). "Synthesis of para-Amino Benzoic Acid–TiO2 Hybrid Nanostructures of Controlled Functionality by an Aqueous One-Step Process". European Journal of Inorganic Chemistry. 2008 (6): 980–987. doi:10.1002/ejic.200700971.
- ^ Gasparro, F. P.; Mitchnick, M.; Nash, J. F. A Review of Sunscreen Safety and Efficacy Photochem. Photobiol. 1998, 68, 243, 256.
- ^ Snyder, Diane Sekura; May, Marian (1975). "Ability Of Paba To Protect Mammalian Skin From Ultraviolet Light-Induced Skin Tumors And Actinic Damage". Journal of Investigative Dermatology. 65 (6): 543–546. doi:10.1111/1523-1747.ep12610349. ISSN 0022-202X.
- ^ Osgood, Pauline J.; Moss, Stephen H.; Davies, David J. G. (1982). "The Sensitization of Near-Ultraviolet Radiation Killing of Mammalian Cells by the Sunscreen Agent Para-aminobenzoic Acid". Journal of Investigative Dermatology. 79 (6): 354–7. doi:10.1111/1523-1747.ep12529409. PMID 6982950.
- ^ "PABA". Safe Cosmetics. Retrieved 2024-11-19.
- ^ US 10064810, Stagg, Amanda M; Rubinson, Emily H., "Matte cosmetic compositions", published September 4, 2018
- ^ Mathias, C. G.; Mailbach, H. I.; Epstein, J. (1978). "Allergic contact photodermatitis to para-aminobenzoic acid". Archives of Dermatology. 114 (11): 1665–1666. ISSN 0003-987X. PMID 309749.
- ^ Toxicity, Local Anesthetics: eMedicine Emergency Medicine