Potassium octachlorodimolybdate

| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| K4[Mo2Cl8] | |
| Molar mass | 631.89 g·mol−1 |
| Appearance | red crystals |
| Density | 2.54 g/cm3 |
| soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
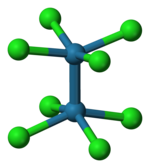
Potassium octachlorodimolybdate (systematically named potassium bis(tetrachloromolybdate)(Mo–Mo)(4−)) is an inorganic compound with the chemical formula K4[Mo2Cl8]. It is known as a red-coloured, microcrystalline solid. The anion is of historic interest as one of the earliest illustrations of a quadruple bonding. The salt is usually obtained as the pink-coloured dihydrate.
The compound is prepared in two steps from molybdenum hexacarbonyl:[1][2]
- 2 Mo(CO)6 + 4 CH3CO2H → (CH3CO2)4Mo2 + 2 H2 + 12 CO
- (CH3CO2)4Mo2 + 4 HCl + 4 KCl → K4[Mo2Cl8] + 4 CH3CO2H
The reaction of the acetate with HCl was first described as providing trimolybdenum compounds,[3] but subsequent crystallographic analysis confirmed that the salt contains the [Cl4Mo≣MoCl4]4− anion, with D4h symmetry, in which the two Mo atoms are linked by a quadruple bond. Each Mo atom is bounded with four Cl− ligands by a single bond. Each MoCl4 group is a regular square pyramid, with an Mo atom at the apex, and four Cl atoms at the vertices of the square base of the pyramid. The Mo–Mo distance is 214 pm.[4]
References
[edit]- ^ Brignole, A. B.; Cotton, F. A.; Dori, Z. (1972). "Rhenium and Molybdenum Compounds Containing Quadruple Bonds". Inorganic Syntheses. Vol. 13. pp. 81–89. doi:10.1002/9780470132449.ch15. ISBN 978-0-470-13244-9.
{{cite book}}:|journal=ignored (help) - ^ Girolami, G. S.; Rauchfuss, T. B.; Angelici, R. J. (1999). Synthesis and Technique in Inorganic Chemistry. Mill Valley, CA: University Science Books. ISBN 978-0-935702-48-4.
- ^ Allison, G. B.; Anderson, I. R.; Sheldon, J. C. (1967). "The Preparation of Halogenotrimolybdate(II) Compounds". Aust. J. Chem. 20 (5): 869–876. doi:10.1071/CH9670869.
- ^ Brencic, Jurij V.; Cotton, F. Albert (1969). "Octachlorodimolybdate(II) Ion. Species with a Quadruple Metal–Metal Bond". Inorg. Chem. 8: 7–10. doi:10.1021/ic50071a002.
