Carbon nanotube


| Part of a series of articles on |
| Nanomaterials |
|---|
 |
| Carbon nanotubes |
| Fullerenes |
| Other nanoparticles |
| Nanostructured materials |
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range (nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized:
- Single-walled carbon nanotubes (SWCNTs) have diameters around 0.5–2.0 nanometres, about 100,000 times smaller than the width of a human hair. They can be idealised as cutouts from a two-dimensional graphene sheet rolled up to form a hollow cylinder.
- Multi-walled carbon nanotubes (MWCNTs) consist of nested single-wall carbon nanotubes in a nested, tube-in-tube structure. Double- and triple-walled carbon nanotubes are special cases of MWCNT.
Carbon nanotubes can exhibit remarkable properties, such as exceptional tensile strength and thermal conductivity because of their nanostructure and strength of the bonds between carbon atoms. Some SWCNT structures exhibit high electrical conductivity while others are semiconductors. In addition, carbon nanotubes can be chemically modified. These properties are expected to be valuable in many areas of technology, such as electronics, optics, composite materials (replacing or complementing carbon fibres), nanotechnology (including nanomedicine), and other applications of materials science.
The predicted properties for SWCNTs were tantalising, but a path to synthesising them was lacking until 1993, when Iijima and Ichihashi at NEC, and Bethune and others at IBM independently discovered that co-vaporising carbon and transition metals such as iron and cobalt could specifically catalyse SWCNT formation. These discoveries triggered research that succeeded in greatly increasing the efficiency of the catalytic production technique, and led to an explosion of work to characterise and find applications for SWCNTs.
History
[edit]The true identity of the discoverers of carbon nanotubes is a subject of some controversy.[1] A 2006 editorial written by Marc Monthioux and Vladimir Kuznetsov in the journal Carbon described the origin of the carbon nanotube.[2] A large percentage of academic and popular literature attributes the discovery of hollow, nanometre-size tubes composed of graphitic carbon to Sumio Iijima of NEC in 1991. His paper initiated a flurry of excitement and could be credited with inspiring the many scientists now studying applications of carbon nanotubes. Though Iijima has been given much of the credit for discovering carbon nanotubes, it turns out that the timeline of carbon nanotubes goes back much further than 1991.[1]
In 1952, L. V. Radushkevich and V. M. Lukyanovich published clear images of 50-nanometre diameter tubes made of carbon in the Journal of Physical Chemistry Of Russia.[3] This discovery was largely unnoticed, as the article was published in Russian, and Western scientists' access to Soviet press was limited during the Cold War. Monthioux and Kuznetsov mentioned in their Carbon editorial:[2]
The fact is, Radushkevich and Lukyanovich [...] should be credited for the discovery that carbon filaments could be hollow and have a nanometre-size diameter, that is to say for the discovery of carbon nanotubes.
In 1976, Morinobu Endo of CNRS observed hollow tubes of rolled up graphite sheets synthesised by a chemical vapour-growth technique.[4] The first specimens observed would later come to be known as single-walled carbon nanotubes (SWNTs).[5] Endo, in his early review of vapor-phase-grown carbon fibers (VPCF), also reminded us that he had observed a hollow tube, linearly extended with parallel carbon layer faces near the fiber core.[6] This appears to be the observation of multi-walled carbon nanotubes at the center of the fiber.[5] The mass-produced MWCNTs today are strongly related to the VPGCF developed by Endo.[5] In fact, they call it the "Endo-process", out of respect for his early work and patents.[5][7] In 1979, John Abrahamson presented evidence of carbon nanotubes at the 14th Biennial Conference of Carbon at Pennsylvania State University. The conference paper described carbon nanotubes as carbon fibers that were produced on carbon anodes during arc discharge. A characterization of these fibers was given, as well as hypotheses for their growth in a nitrogen atmosphere at low pressures.[8]
In 1981, a group of Soviet scientists published the results of chemical and structural characterization of carbon nanoparticles produced by a thermocatalytic disproportionation of carbon monoxide. Using TEM images and XRD patterns, the authors suggested that their "carbon multi-layer tubular crystals" were formed by rolling graphene layers into cylinders. They speculated that via this rolling, many different arrangements of graphene hexagonal nets are possible. They suggested two such possible arrangements: a circular arrangement (armchair nanotube); and a spiral, helical arrangement (chiral tube).[9]
In 1987, Howard G. Tennent of Hyperion Catalysis was issued a U.S. patent for the production of "cylindrical discrete carbon fibrils" with a "constant diameter between about 3.5 and about 70 nanometers..., length 102 times the diameter, and an outer region of multiple essentially continuous layers of ordered carbon atoms and a distinct inner core...."[10]
Helping to create the initial excitement associated with carbon nanotubes were Iijima's 1991 discovery of multi-walled carbon nanotubes in the insoluble material of arc-burned graphite rods;[11] and Mintmire, Dunlap, and White's independent prediction that if single-walled carbon nanotubes could be made, they would exhibit remarkable conducting properties.[12] Nanotube research accelerated greatly following the independent discoveries[13][14] by Iijima and Ichihashi at NEC and Bethune et al. at IBM of methods to specifically produce single-walled carbon nanotubes by adding transition-metal catalysts to the carbon in an arc discharge. Thess et al.[15] refined this catalytic method by vaporizing the carbon/transition-metal combination in a high-temperature furnace, which greatly improved the yield and purity of the SWNTs and made them widely available for characterization and application experiments. The arc discharge technique, well known to produce the famed Buckminsterfullerene,[16][failed verification] thus played a role in the discoveries of both multi- and single-wall nanotubes, extending the run of serendipitous discoveries relating to fullerenes. The discovery of nanotubes remains a contentious issue. Many believe that Iijima's report in 1991 is of particular importance because it brought carbon nanotubes into the awareness of the scientific community as a whole.[1][5]
In 2020, during an archaeological excavation of Keezhadi in Tamil Nadu, India, ~2600-year-old pottery was discovered whose coatings appear to contain carbon nanotubes. The robust mechanical properties of the nanotubes are partially why the coatings have lasted for so many years, say the scientists.[17]
Structure of SWCNTs
[edit]Basic details
[edit]

The structure of an ideal (infinitely long) single-walled carbon nanotube is that of a regular hexagonal lattice drawn on an infinite cylindrical surface, whose vertices are the positions of the carbon atoms. Since the length of the carbon-carbon bonds is fairly fixed, there are constraints on the diameter of the cylinder and the arrangement of the atoms on it.[18]
In the study of nanotubes, one defines a zigzag path on a graphene-like lattice as a path that turns 60 degrees, alternating left and right, after stepping through each bond. It is also conventional to define an armchair path as one that makes two left turns of 60 degrees followed by two right turns every four steps. On some carbon nanotubes, there is a closed zigzag path that goes around the tube. One says that the tube is of the zigzag type or configuration, or simply is a zigzag nanotube. If the tube is instead encircled by a closed armchair path, it is said to be of the armchair type, or an armchair nanotube. An infinite nanotube that is of one type consists entirely of closed paths of that type, connected to each other.
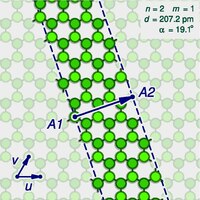
The zigzag and armchair configurations are not the only structures that a single-walled nanotube can have. To describe the structure of a general infinitely long tube, one should imagine it being sliced open by a cut parallel to its axis, that goes through some atom A, and then unrolled flat on the plane, so that its atoms and bonds coincide with those of an imaginary graphene sheet—more precisely, with an infinitely long strip of that sheet. The two halves of the atom A will end up on opposite edges of the strip, over two atoms A1 and A2 of the graphene. The line from A1 to A2 will correspond to the circumference of the cylinder that went through the atom A, and will be perpendicular to the edges of the strip. In the graphene lattice, the atoms can be split into two classes, depending on the directions of their three bonds. Half the atoms have their three bonds directed the same way, and half have their three bonds rotated 180 degrees relative to the first half. The atoms A1 and A2, which correspond to the same atom A on the cylinder, must be in the same class. It follows that the circumference of the tube and the angle of the strip are not arbitrary, because they are constrained to the lengths and directions of the lines that connect pairs of graphene atoms in the same class.
Let u and v be two linearly independent vectors that connect the graphene atom A1 to two of its nearest atoms with the same bond directions. That is, if one numbers consecutive carbons around a graphene cell with C1 to C6, then u can be the vector from C1 to C3, and v be the vector from C1 to C5. Then, for any other atom A2 with same class as A1, the vector from A1 to A2 can be written as a linear combination n u + m v, where n and m are integers. And, conversely, each pair of integers (n,m) defines a possible position for A2.[18] Given n and m, one can reverse this theoretical operation by drawing the vector w on the graphene lattice, cutting a strip of the latter along lines perpendicular to w through its endpoints A1 and A2, and rolling the strip into a cylinder so as to bring those two points together. If this construction is applied to a pair (k,0), the result is a zigzag nanotube, with closed zigzag paths of 2k atoms. If it is applied to a pair (k,k), one obtains an armchair tube, with closed armchair paths of 4k atoms.
Types
[edit]The structure of the nanotube is not changed if the strip is rotated by 60 degrees clockwise around A1 before applying the hypothetical reconstruction above. Such a rotation changes the corresponding pair (n,m) to the pair (−2m,n+m). It follows that many possible positions of A2 relative to A1 — that is, many pairs (n,m) — correspond to the same arrangement of atoms on the nanotube. That is the case, for example, of the six pairs (1,2), (−2,3), (−3,1), (−1,−2), (2,−3), and (3,−1). In particular, the pairs (k,0) and (0,k) describe the same nanotube geometry. These redundancies can be avoided by considering only pairs (n,m) such that n > 0 and m ≥ 0; that is, where the direction of the vector w lies between those of u (inclusive) and v (exclusive). It can be verified that every nanotube has exactly one pair (n,m) that satisfies those conditions, which is called the tube's type. Conversely, for every type there is a hypothetical nanotube. In fact, two nanotubes have the same type if and only if one can be conceptually rotated and translated so as to match the other exactly. Instead of the type (n,m), the structure of a carbon nanotube can be specified by giving the length of the vector w (that is, the circumference of the nanotube), and the angle α between the directions of u and w, may range from 0 (inclusive) to 60 degrees clockwise (exclusive). If the diagram is drawn with u horizontal, the latter is the tilt of the strip away from the vertical.
Chirality and mirror symmetry
[edit]A nanotube is chiral if it has type (n,m), with m > 0 and m ≠ n; then its enantiomer (mirror image) has type (m,n), which is different from (n,m). This operation corresponds to mirroring the unrolled strip about the line L through A1 that makes an angle of 30 degrees clockwise from the direction of the u vector (that is, with the direction of the vector u+v). The only types of nanotubes that are achiral are the (k,0) "zigzag" tubes and the (k,k) "armchair" tubes. If two enantiomers are to be considered the same structure, then one may consider only types (n,m) with 0 ≤ m ≤ n and n > 0. Then the angle α between u and w, which may range from 0 to 30 degrees (inclusive both), is called the "chiral angle" of the nanotube.
Circumference and diameter
[edit]From n and m one can also compute the circumference c, which is the length of the vector w, which turns out to be:
in picometres. The diameter of the tube is then , that is
also in picometres. (These formulas are only approximate, especially for small n and m where the bonds are strained; and they do not take into account the thickness of the wall.)
The tilt angle α between u and w and the circumference c are related to the type indices n and m by:
where arg(x,y) is the clockwise angle between the X-axis and the vector (x,y); a function that is available in many programming languages as atan2(y,x). Conversely, given c and α, one can get the type (n,m) by the formulas:
which must evaluate to integers.
Physical limits
[edit]Narrowest examples
[edit]If n and m are too small, the structure described by the pair (n,m) will describe a molecule that cannot be reasonably called a "tube", and may not even be stable. For example, the structure theoretically described by the pair (1,0) (the limiting "zigzag" type) would be just a chain of carbons. That is a real molecule, the carbyne; which has some characteristics of nanotubes (such as orbital hybridization, high tensile strength, etc.) — but has no hollow space, and may not be obtainable as a condensed phase. The pair (2,0) would theoretically yield a chain of fused 4-cycles; and (1,1), the limiting "armchair" structure, would yield a chain of bi-connected 4-rings. These structures may not be realizable.
The thinnest carbon nanotube proper is the armchair structure with type (2,2), which has a diameter of 0.3 nm. This nanotube was grown inside a multi-walled carbon nanotube. Assigning of the carbon nanotube type was done by a combination of high-resolution transmission electron microscopy (HRTEM), Raman spectroscopy, and density functional theory (DFT) calculations.[19]
The thinnest freestanding single-walled carbon nanotube is about 0.43 nm in diameter.[20] Researchers suggested that it can be either (5,1) or (4,2) SWCNT, but the exact type of the carbon nanotube remains questionable.[21] (3,3), (4,3), and (5,1) carbon nanotubes (all about 0.4 nm in diameter) were unambiguously identified using aberration-corrected high-resolution transmission electron microscopy inside double-walled CNTs.[22]
Length
[edit]The observation of the longest carbon nanotubes grown so far, around 0.5 metre (550 mm) long, was reported in 2013.[23] These nanotubes were grown on silicon substrates using an improved chemical vapor deposition (CVD) method and represent electrically uniform arrays of single-walled carbon nanotubes.[24]
The shortest carbon nanotube can be considered to be the organic compound cycloparaphenylene, which was synthesized in 2008 by Ramesh Jasti.[25] Other small molecule carbon nanotubes have been synthesized since.[26]
Density
[edit]The highest density of CNTs was achieved in 2013, grown on a conductive titanium-coated copper surface that was coated with co-catalysts cobalt and molybdenum at lower than typical temperatures of 450 °C. The tubes averaged a height of 380 nm and a mass density of 1.6 g cm−3. The material showed ohmic conductivity (lowest resistance ~22 kΩ).[27][28]
Variants
[edit]There is no consensus on some terms describing carbon nanotubes in the scientific literature: both "-wall" and "-walled" are being used in combination with "single", "double", "triple", or "multi", and the letter C is often omitted in the abbreviation, for example, multi-walled carbon nanotube (MWNT). The International Standards Organization typically uses "single-walled carbon nanotube (SWCNT)" or "multi-walled carbon nanotube (MWCNT)" in its documents.[29]
Multi-walled
[edit]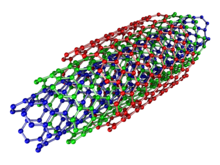
Multi-walled nanotubes (MWNTs) consist of multiple rolled layers (concentric tubes) of graphene. There are two models that can be used to describe the structures of multi-walled nanotubes. In the Russian Doll model, sheets of graphite are arranged in concentric cylinders, e.g., a (0,8) single-walled nanotube (SWNT) within a larger (0,17) single-walled nanotube. In the Parchment model, a single sheet of graphite is rolled in around itself, resembling a scroll of parchment or a rolled newspaper. The interlayer distance in multi-walled nanotubes is close to the distance between graphene layers in graphite, approximately 3.4 Å. The Russian Doll structure is observed more commonly. Its individual shells can be described as SWNTs, which can be metallic or semiconducting.[30][31] Because of statistical probability and restrictions on the relative diameters of the individual tubes, one of the shells, and thus the whole MWNT, is usually a zero-gap metal.[32]
Double-walled carbon nanotubes (DWNTs) form a special class of nanotubes because their morphology and properties are similar to those of SWNTs but they are more resistant to attacks by chemicals.[33] This is especially important when it is necessary to graft chemical functions to the surface of the nanotubes (functionalization) to add properties to the CNT. Covalent functionalization of SWNTs will break some C=C double bonds, leaving "holes" in the structure on the nanotube and thus modifying both its mechanical and electrical properties. In the case of DWNTs, only the outer wall is modified. DWNT synthesis on the gram-scale by the CCVD technique was first proposed in 2003[34] from the selective reduction of oxide solutions in methane and hydrogen.
The telescopic motion ability of inner shells, allowing them to act as low-friction, low-wear nanobearings and nanosprings, may make them a desirable material in nanoelectromechanical systems (NEMS) .[35] The retraction force that occurs to telescopic motion is caused by the Lennard-Jones interaction between shells, and its value is about 1.5 nN.[36]
Junctions and crosslinking
[edit]
Junctions between two or more nanotubes have been widely discussed theoretically.[37][38] Such junctions are quite frequently observed in samples prepared by arc discharge as well as by chemical vapor deposition. The electronic properties of such junctions were first considered theoretically by Lambin et al.,[39] who pointed out that a connection between a metallic tube and a semiconducting one would represent a nanoscale heterojunction. Such a junction could therefore form a component of a nanotube-based electronic circuit. The adjacent image shows a junction between two multiwalled nanotubes.
Junctions between nanotubes and graphene have been considered theoretically[40] and studied experimentally.[41] Nanotube-graphene junctions form the basis of pillared graphene, in which parallel graphene sheets are separated by short nanotubes.[42] Pillared graphene represents a class of three-dimensional carbon nanotube architectures.

Recently, several studies have highlighted the prospect of using carbon nanotubes as building blocks to fabricate three-dimensional macroscopic (>100 nm in all three dimensions) all-carbon devices. Lalwani et al. have reported a novel radical-initiated thermal crosslinking method to fabricate macroscopic, free-standing, porous, all-carbon scaffolds using single- and multi-walled carbon nanotubes as building blocks.[43] These scaffolds possess macro-, micro-, and nano-structured pores, and the porosity can be tailored for specific applications. These 3D all-carbon scaffolds/architectures may be used for the fabrication of the next generation of energy storage, supercapacitors, field emission transistors, high-performance catalysis, photovoltaics, and biomedical devices, implants, and sensors.[44][45]
Other morphologies
[edit]Carbon nanobuds are a newly created material combining two previously discovered allotropes of carbon: carbon nanotubes and fullerenes. In this new material, fullerene-like "buds" are covalently bonded to the outer sidewalls of the underlying carbon nanotube. This hybrid material has useful properties of both fullerenes and carbon nanotubes. In particular, they have been found to be exceptionally good field emitters.[46] In composite materials, the attached fullerene molecules may function as molecular anchors preventing slipping of the nanotubes, thus improving the composite's mechanical properties.
A carbon peapod[47][48] is a novel hybrid carbon material which traps fullerene inside a carbon nanotube. It can possess interesting magnetic properties with heating and irradiation. It can also be applied as an oscillator during theoretical investigations and predictions.[49][50]
In theory, a nanotorus is a carbon nanotube bent into a torus (doughnut shape). Nanotori are predicted to have many unique properties, such as magnetic moments 1000 times larger than that previously expected for certain specific radii.[51] Properties such as magnetic moment, thermal stability, etc. vary widely depending on the radius of the torus and the radius of the tube.[51][52]
Graphenated carbon nanotubes are a relatively new hybrid that combines graphitic foliates grown along the sidewalls of multiwalled or bamboo-style CNTs. The foliate density can vary as a function of deposition conditions (e.g., temperature and time) with their structure ranging from a few layers of graphene (< 10) to thicker, more graphite-like.[53] The fundamental advantage of an integrated graphene-CNT structure is the high surface area three-dimensional framework of the CNTs coupled with the high edge density of graphene. Depositing a high density of graphene foliates along the length of aligned CNTs can significantly increase the total charge capacity per unit of nominal area as compared to other carbon nanostructures.[54]
Cup-stacked carbon nanotubes (CSCNTs) differ from other quasi-1D carbon structures, which normally behave as quasi-metallic conductors of electrons. CSCNTs exhibit semiconducting behavior because of the stacking microstructure of graphene layers.[55]
Properties
[edit]Many properties of single-walled carbon nanotubes depend significantly on the (n,m) type, and this dependence is non-monotonic (see Kataura plot). In particular, the band gap can vary from zero to about 2 eV and the electrical conductivity can show metallic or semiconducting behavior.
Mechanical
[edit]
Carbon nanotubes are the strongest and stiffest materials yet discovered in terms of tensile strength and elastic modulus. This strength results from the covalent sp2 bonds formed between the individual carbon atoms. In 2000, a multiwalled carbon nanotube was tested to have a tensile strength of 63 GPa (9,100,000 psi).[56] (For illustration, this translates into the ability to endure tension of a weight equivalent to 6,422 kilograms-force (62,980 N; 14,160 lbf) on a cable with cross-section of 1 mm2 (0.0016 sq in)). Further studies, such as one conducted in 2008, revealed that individual CNT shells have strengths of up to ≈100 GPa (15,000,000 psi), which is in agreement with quantum/atomistic models.[57] Because carbon nanotubes have a low density for a solid of 1.3 to 1.4 g/cm3,[58] its specific strength of up to 48,000 kN·m/kg is the best of known materials, compared to high-carbon steel's 154 kN·m/kg.
Although the strength of individual CNT shells is extremely high, weak shear interactions between adjacent shells and tubes lead to significant reduction in the effective strength of multiwalled carbon nanotubes and carbon nanotube bundles down to only a few GPa.[59] This limitation has been recently addressed by applying high-energy electron irradiation, which crosslinks inner shells and tubes, and effectively increases the strength of these materials to ≈60 GPa for multiwalled carbon nanotubes[57] and ≈17 GPa for double-walled carbon nanotube bundles.[59] CNTs are not nearly as strong under compression. Because of their hollow structure and high aspect ratio, they tend to undergo buckling when placed under compressive, torsional, or bending stress.[60]
On the other hand, there is evidence that in the radial direction they are rather soft. The first transmission electron microscope observation of radial elasticity suggested that even van der Waals forces can deform two adjacent nanotubes. Later, nanoindentations with an atomic force microscope were performed by several groups to quantitatively measure the radial elasticity of multiwalled carbon nanotubes and tapping/contact mode atomic force microscopy was also performed on single-walled carbon nanotubes. Their high Young's modulus in the linear direction, of on the order of several GPa (and even up to an experimentally-measured 1.8 TPa, for nanotubes near 2.4 μm in length[61]), further suggests they may be soft in the radial direction.
Electrical
[edit]
Unlike graphene, which is a two-dimensional semimetal, carbon nanotubes are either metallic[62] or semiconducting along the tubular axis. For a given (n,m) nanotube, if n = m, the nanotube is metallic; if n − m is a multiple of 3 and n ≠ m, then the nanotube is quasi-metallic with a very small band gap, otherwise the nanotube is a moderate semiconductor.[63] Thus, all armchair (n = m) nanotubes are metallic, and nanotubes (6,4), (9,1), etc. are semiconducting.[64] Carbon nanotubes are not semimetallic because the degenerate point (the point where the π [bonding] band meets the π* [anti-bonding] band, at which the energy goes to zero) is slightly shifted away from the K point in the Brillouin zone because of the curvature of the tube surface, causing hybridization between the σ* and π* anti-bonding bands, modifying the band dispersion.
The rule regarding metallic versus semiconductor behavior has exceptions because curvature effects in small-diameter tubes can strongly influence electrical properties. Thus, a (5,0) SWCNT that should be semiconducting in fact is metallic according to the calculations. Likewise, zigzag and chiral SWCNTs with small diameters that should be metallic have a finite gap (armchair nanotubes remain metallic).[64] In theory, metallic nanotubes can carry an electric current density of 4 × 109 A/cm2, which is more than 1,000 times greater than those of metals such as copper,[65] where for copper interconnects, current densities are limited by electromigration. Carbon nanotubes are thus being explored as interconnects and conductivity-enhancing components in composite materials, and many groups are attempting to commercialize highly conducting electrical wire assembled from individual carbon nanotubes. There are significant challenges to be overcome however, such as undesired current saturation under voltage,[66] and the much more resistive nanotube-to-nanotube junctions and impurities, all of which lower the electrical conductivity of the macroscopic nanotube wires by orders of magnitude, as compared to the conductivity of the individual nanotubes.
Because of its nanoscale cross-section, electrons propagate only along the tube's axis. As a result, carbon nanotubes are frequently referred to as one-dimensional conductors. The maximum electrical conductance of a single-walled carbon nanotube is 2G0, where G0 = 2e2/h is the conductance of a single ballistic quantum channel.[67]
Because of the role of the π-electron system in determining the electronic properties of graphene, doping in carbon nanotubes differs from that of bulk crystalline semiconductors from the same group of the periodic table (e.g., silicon). Graphitic substitution of carbon atoms in the nanotube wall by boron or nitrogen dopants leads to p-type and n-type behavior, respectively, as would be expected in silicon. However, some non-substitutional (intercalated or adsorbed) dopants introduced into a carbon nanotube, such as alkali metals and electron-rich metallocenes, result in n-type conduction because they donate electrons to the π-electron system of the nanotube. By contrast, π-electron acceptors such as FeCl3 or electron-deficient metallocenes function as p-type dopants because they draw π-electrons away from the top of the valence band.
Intrinsic superconductivity has been reported,[68][69][70] although other experiments found no evidence of this, leaving the claim a subject of debate.[71]
In 2021, Michael Strano, the Carbon P. Dubbs Professor of Chemical Engineering at MIT, published department findings on the use of carbon nanotubes to create an electric current.[72] By immersing the structures in an organic solvent, the liquid drew electrons out of the carbon particles. Strano was quoted as saying, "This allows you to do electrochemistry, but with no wires," and represents a significant breakthrough in the technology.[73] Future applications include powering micro- or nanoscale robots, as well as driving alcohol oxidation reactions, which are important in the chemicals industry.[73]
Crystallographic defects also affect the tube's electrical properties. A common result is lowered conductivity through the defective region of the tube. A defect in metallic armchair-type tubes (which can conduct electricity) can cause the surrounding region to become semiconducting, and single monatomic vacancies induce magnetic properties.[74]
Electromechanical
[edit]Semiconducting carbon nanotubes have shown piezoresistive property when applying mechanical force. The structural deformation causes a change in the band gap which effects the conductance. This property has the potential to be used in strain sensors.[75][76]
Optical
[edit]Carbon nanotubes have useful absorption, photoluminescence (fluorescence), and Raman spectroscopy properties. Spectroscopic methods offer the possibility of quick and non-destructive characterization of relatively large amounts of carbon nanotubes. There is a strong demand for such characterization from the industrial point of view: numerous parameters of nanotube synthesis can be changed, intentionally or unintentionally, to alter the nanotube quality, such as the non-tubular carbon content, structure (chirality) of the produced nanotubes, and structural defects. These features then determine nearly all other significant optical, mechanical, and electrical properties.
Carbon nanotube optical properties have been explored for use in applications such as for light-emitting diodes (LEDs)[77][78] and photo-detectors[79] based on a single nanotube have been produced in the lab. Their unique feature is not the efficiency, which is yet relatively low, but the narrow selectivity in the wavelength of emission and detection of light and the possibility of its fine-tuning through the nanotube structure. In addition, bolometer[80] and optoelectronic memory[81] devices have been realised on ensembles of single-walled carbon nanotubes. Nanotube fluorescence has been investigated for the purposes of imaging and sensing in biomedical applications.[82][83][84]
Thermal
[edit]All nanotubes are expected to be very good thermal conductors along the tube,[85][86] exhibiting a property known as "ballistic conduction", but good insulators lateral to the tube axis. Measurements show that an individual SWNT has a room-temperature thermal conductivity along its axis of about 3500 W·m−1·K−1;[87] compare this to copper, a metal well known for its good thermal conductivity, which transmits 385 W·m−1·K−1. An individual SWNT has a room-temperature thermal conductivity lateral to its axis (in the radial direction) of about 1.52 W·m−1·K−1,[88] which is about as thermally conductive as soil. Macroscopic assemblies of nanotubes such as films or fibres have reached up to 1500 W·m−1·K−1 so far.[89] Networks composed of nanotubes demonstrate different values of thermal conductivity, from the level of thermal insulation with the thermal conductivity of 0.1 W·m−1·K−1 to such high values.[90] That is dependent on the amount of contribution to the thermal resistance of the system caused by the presence of impurities, misalignments and other factors. The temperature stability of carbon nanotubes is estimated to be up to 2800 °C in vacuum and about 750 °C in air.[91]
Crystallographic defects strongly affect the tube's thermal properties. Such defects lead to phonon scattering, which in turn increases the relaxation rate of the phonons. This reduces the mean free path and reduces the thermal conductivity of nanotube structures. Phonon transport simulations indicate that substitutional defects such as nitrogen or boron will primarily lead to the scattering of high-frequency optical phonons. However, larger-scale defects such as Stone–Wales defects cause phonon scattering over a wide range of frequencies, leading to a greater reduction in thermal conductivity.[92]

Antibacterial
[edit]Recently, carbon-nanotubes have been shown to have antibacterial properties. They disrupt normal bacterial function by causing physical/mechanical damage, facilitating oxidative stress or lipid extraction, inhibiting bacterial metabolism, and isolating functional sites via wrapping with CNM-containing nanomaterials.[93]
Synthesis
[edit]Techniques have been developed to produce nanotubes in sizeable quantities, including arc discharge, laser ablation, chemical vapor deposition (CVD) and high-pressure carbon monoxide disproportionation (HiPCO). Among these arc discharge, laser ablation are batch by batch process, Chemical Vapor Deposition can be used both for batch by batch or continuous processes,[94][95] and HiPCO is gas phase continuous process.[96] Most of these processes take place in a vacuum or with process gases. The CVD growth method is popular, as it yields high quantity and has a degree of control over diameter, length and morphology. Using particulate catalysts, large quantities of nanotubes can be synthesized by these methods, and industrialisation is well on its way, with several CNT and CNT fibers factory around the world. One problem of CVD processes is the high variability in the nanotube's characteristics [97] The HiPCO process advances in catalysis and continuous growth are making CNTs more commercially viable.[98] The HiPCO process helps in producing high purity single-walled carbon nanotubes in higher quantity. The HiPCO reactor operates at high temperature 900-1100 °C and high pressure ~30-50 bar.[99] It uses carbon monoxide as the carbon source and iron pentacarbonyl or nickel tetracarbonyl as a catalyst. These catalysts provide a nucleation site for the nanotubes to grow,[96] while cheaper iron-based catalysts like Ferrocene can be used for CVD process.
Vertically aligned carbon nanotube arrays are also grown by thermal chemical vapor deposition. A substrate (quartz, silicon, stainless steel, carbon fibers, etc.) is coated with a catalytic metal (Fe, Co, Ni) layer. Typically that layer is iron and is deposited via sputtering to a thickness of 1–5 nm. A 10–50 nm underlayer of alumina is often also put down on the substrate first. This imparts controllable wetting and good interfacial properties. When the substrate is heated to the growth temperature (~600 to 850 °C), the continuous iron film breaks up into small islands with each island then nucleating a carbon nanotube. The sputtered thickness controls the island size and this in turn determines the nanotube diameter. Thinner iron layers drive down the diameter of the islands and drive down the diameter of the nanotubes grown. The amount of time the metal island can sit at the growth temperature is limited as they are mobile and can merge into larger (but fewer) islands. Annealing at the growth temperature reduces the site density (number of CNT/mm2) while increasing the catalyst diameter.
The as-prepared carbon nanotubes always have impurities such as other forms of carbon (amorphous carbon, fullerene, etc.) and non-carbonaceous impurities (metal used for catalyst).[100][101] These impurities need to be removed to make use of the carbon nanotubes in applications.[102]
Purification
[edit]As-synthesized carbon nanotubes typically contain impurities and most importantly different chiralities of carbon nanotubes. Therefore, multiple methods have been developed to purify them including polymer-assisted,[103][104][105] density gradient ultracentrifugation (DGU),[106][107] chromatography [108][109][110] and aqueous two-phase extraction (ATPE).[111][112][113][114] These methods have been reviewed in multiple articles.[115][116][117]
Certain polymers selectively disperse or wrap CNTs of a particular chirality, metallic character or diameter. For example, poly(phenylenevinylenes) disperses CNTs of specific diameters (0.75–0.84 nm) and polyfluorenes are highly selective for semiconducting CNTs. It involves mainly two steps, sonicate the mixture (CNTs and polymers in solvent), centrifuge and the supernatant are desired CNTs.
Density gradient ultracentrifugation is a method based on the density difference of CNTs, so that different components are layered in centrifuge tubes under centrifugal force. Chromatography-based methods include size exclusion (SEC), ion-exchange (IEX) and gel chromatography. For SEC, CNTs are separated due to the difference in size using a stationary phase with different pore size. As for IEX, the separation is achieved based on their differential adsorption and desorption onto chemically functionalized resins packed in an IEX column, so understanding the interaction between CNTs mixtures and resins is important. The first IEX is reported to separate DNA-SWCNTs.[118] Gel chromatography is based on the partition of CNTs between stationary and mobile phase, it's found semiconducting CNTs are more strongly attracted by gel than metallic CNTs.[119][120] While it shows potential, the current application is limited to the separation of semiconducting (n,m) species.
ATPE uses two water-soluble polymers such as polyethylene glycol (PEG) and dextran. When mixed, two immiscible aqueous phases form spontaneously, and each of the two phases shows a different affinity to CNTs. Partition depends on the solvation energy difference between two similar phases of microscale volumes. By changing the separation system or temperatures, and adding strong oxidants, reductants, or salts, the partition of CNTs species into the two phases can be adjusted.
Despite the progress that has been made to separate and purify CNTs, many challenges remain, such as the growth of chirality-controlled CNTs, so that no further purification is needed, or large-scale purification.
Advantages of monochiral CNTs
[edit]Monochiral CNTs have the advantage that they do contain less or no impurities, well-defined non-congested optical spectra. This allows to create for example CNT-based biosensors with higher sensitivity and selectivity.[121] For example, monochiral SWCNTs are necessary for multiplexed and ratiometric sensing schemes,[122][123] enhanced sensitivity [124] of biocompatibility.[125]
Functionalization
[edit]Carbon nanotubes can be functionalized to attain desired properties that can be used in a wide variety of applications.[126] The two main methods of carbon nanotube functionalization are covalent and non-covalent modifications. Because of their apparent hydrophobic nature,[127] carbon nanotubes tend to agglomerate hindering their dispersion in solvents or viscous polymer melts. The resulting nanotube bundles or aggregates reduce the mechanical performance of the final composite. The surface of the carbon nanotubes can be modified to reduce the hydrophobicity and improve interfacial adhesion to a bulk polymer through chemical attachment.[128]
Chemical routes such as covalent functionalization have been studied extensively, which involves the oxidation of CNTs via strong acids (e.g. sulfuric acid, nitric acid, or a mixture of both) in order to set the carboxylic groups onto the surface of the CNTs as the final product or for further modification by esterification or amination. Free radical grafting is a promising technique among covalent functionalization methods, in which alkyl or aryl peroxides, substituted anilines, and diazonium salts are used as the starting agents.
Functionalization can improve CNTs characteristically weak dispersibility in many solvents, such as water - a consequence of their strong intermolecular p–p interactions. This can enhance the processing and manipulation of insoluble CNTs, rendering them useful for synthesizing innovative CNT nanofluids with impressive properties that are tunable for a wide range of applications.
Free radical grafting of macromolecules (as the functional group) onto the surface of CNTs can improve the solubility of CNTs compared to common acid treatments which involve the attachment of small molecules such as hydroxyl onto the surface of CNTs. The solubility of CNTs can be improved significantly by free-radical grafting because the large functional molecules facilitate the dispersion of CNTs in a variety of solvents even at a low degree of functionalization. Recently an innovative environmentally friendly approach has been developed for the covalent functionalization of multi-walled carbon nanotubes (MWCNTs) using clove buds. This approach is innovative and green because it does not use toxic and hazardous acids which are typically used in common carbon nanomaterial functionalization procedures. The MWCNTs are functionalized in one pot using a free radical grafting reaction. The clove-functionalized MWCNTs are then dispersed in water producing a highly stable multi-walled carbon nanotube aqueous suspension (nanofluids).[129]
The surface of carbon nanotubes can be chemically modified by coating spinel nanoparticles by hydrothermal synthesis[130] and can be used for water oxidation purposes.[131]
In addition, the surface of carbon nanotubes can be fluorinated or halofluorinated by heating while in contact with a fluoroorganic substance, thereby forming partially fluorinated carbons (so-called Fluocar materials) with grafted (halo)fluoroalkyl functionality.[132][133]
Modeling
[edit]
Carbon nanotubes are modelled in a similar manner as traditional composites in which a reinforcement phase is surrounded by a matrix phase. Ideal models such as cylindrical, hexagonal and square models are common. The size of the micromechanics model is highly function of the studied mechanical properties. The concept of representative volume element (RVE) is used to determine the appropriate size and configuration of the computer model to replicate the actual behavior of the CNT-reinforced nanocomposite. Depending on the material property of interest (thermal, electrical, modulus, creep), one RVE might predict the property better than the alternatives. While the implementation of the ideal model is computationally efficient, they do not represent microstructural features observed in scanning electron microscopy of actual nanocomposites. To incorporate realistic modeling, computer models are also generated to incorporate variability such as waviness, orientation and agglomeration of multiwall or single-wall carbon nanotubes.[134]
Metrology
[edit]There are many metrology standards and reference materials available for carbon nanotubes.[135]
For single-wall carbon nanotubes, ISO/TS 10868 describes a measurement method for the diameter, purity, and fraction of metallic nanotubes through optical absorption spectroscopy,[136] while ISO/TS 10797 and ISO/TS 10798 establish methods to characterize the morphology and elemental composition of single-wall carbon nanotubes, using transmission electron microscopy and scanning electron microscopy respectively, coupled with energy dispersive X-ray spectrometry analysis.[137][138]
NIST SRM 2483 is a soot of single-wall carbon nanotubes used as a reference material for elemental analysis, and was characterized using thermogravimetric analysis, prompt gamma activation analysis, induced neutron activation analysis, inductively coupled plasma mass spectroscopy, resonant Raman scattering, UV-visible-near infrared fluorescence spectroscopy and absorption spectroscopy, scanning electron microscopy, and transmission electron microscopy.[139][140] The Canadian National Research Council also offers a certified reference material SWCNT-1 for elemental analysis using neutron activation analysis and inductively coupled plasma mass spectroscopy.[135][141] NIST RM 8281 is a mixture of three lengths of single-wall carbon nanotube.[139][142]
For multiwall carbon nanotubes, ISO/TR 10929 identifies the basic properties and the content of impurities,[143] while ISO/TS 11888 describes morphology using scanning electron microscopy, transmission electron microscopy, viscometry, and light scattering analysis.[144] ISO/TS 10798 is also valid for multiwall carbon nanotubes.[138]
Safety and health
[edit]
The National Institute for Occupational Safety and Health (NIOSH) is the leading United States federal agency conducting research and providing guidance on the occupational safety and health implications and applications of nanomaterials. Early scientific studies have indicated that nanoscale particles may pose a greater health risk than bulk materials due to a relative increase in surface area per unit mass. Increase in length and diameter of CNT is correlated to increased toxicity[145] and pathological alterations in lung.[146] The biological interactions of nanotubes are not well understood, and the field is open to continued toxicological studies. It is often difficult to separate confounding factors, and since carbon is relatively biologically inert, some of the toxicity attributed to carbon nanotubes may be instead due to residual metal catalyst contamination. In previous studies, only Mitsui-7 was reliably demonstrated to be carcinogenic, although for unclear/unknown reasons.[147] Unlike many common mineral fibers (such as asbestos), most SWCNTs and MWCNTs do not fit the size and aspect-ratio criteria to be classified as respirable fibers. In 2013, given that the long-term health effects have not yet been measured, NIOSH published a Current Intelligence Bulletin[148] detailing the potential hazards and recommended exposure limit for carbon nanotubes and fibers.[149] The U.S. National Institute for Occupational Safety and Health has determined non-regulatory recommended exposure limits (RELs) of 1 μg/m3 for carbon nanotubes and carbon nanofibers as background-corrected elemental carbon as an 8-hour time-weighted average (TWA) respirable mass concentration.[150] Although CNT caused pulmonary inflammation and toxicity in mice, exposure to aerosols generated from sanding of composites containing polymer-coated MWCNTs, representative of the actual end-product, did not exert such toxicity.[151]
As of October 2016, single-wall carbon nanotubes have been registered through the European Union's Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulations, based on evaluation of the potentially hazardous properties of SWCNT. Based on this registration, SWCNT commercialization is allowed in the EU up to 100 metric tons.[152] Currently, the type of SWCNT registered through REACH is limited to the specific type of single-wall carbon nanotubes manufactured by OCSiAl, which submitted the application.[153]
Applications
[edit]
Carbon nanotubes are currently used in multiple industrial and consumer applications. These include battery components, polymer composites, to improve the mechanical, thermal and electrical properties of the bulk product, and as a highly absorptive black paint. Many other applications are under development, including field effect transistors for electronics, high-strength fabrics, biosensors for biomedical and agricultural applications, and many others.
Biomedical Applications
[edit]Because of their relatively large surface area, CNTs are capable of interacting with a wide variety of therapeutic and diagnostic agents (drugs, genes, vaccines, antibodies, biosensors, etc.). This can be utilized to assist in drug delivery directly into cells. [154]In addition, CNTs have recently been used as reinforcements in implants and scaffolds due to their suitable reaction area, high elastic modulus, and load transfer capability.[155][156]

 PMCID: PMC11085746
PMCID: PMC11085746 
CNTs have been shown to increase the effectiveness of bioactive coatings for the attachment, proliferation, and differentiation of osteoblasts, and has been used as a bone substitution material.[157]
CNTs may be used as reinforcing materials for chitosan-containing coatings used on implants and medical scaffolds.[158]
Biosensing
[edit]SWCNTs have nanoscale dimensions that fit to the size of biological species. Due to this size compatibility and their large surface-to-volume ratio, they are sensitive to changes in their chemical environment.[159][160] Through covalent and non-covalent surface functionalization, SWCNTs can be precisely tailored for selective molecular interactions with a target analyte.[121] The SWCNT represents the transduction unit that converts the interaction into a signal change (optical or electrical). Due to continuous progress in the development of detection strategies, there are numerous examples of the use of SWCNTs as highly sensitive nanosensors (even down to the single molecule level[161][162][163]) for a variety of important biomolecules. Examples include the detection of reactive oxygen and nitrogen species,[164][165][166][167] neurotransmitters,[163][168][169][170][124] other small molecules,[171][172][173] lipids,[174][175] proteins,[176][177] sugars,[178][179] DNA/RNA,[180][181] enzymes[182][183] as well as bacteria.[184]

The signal change manifests itself in an increase or decrease in the current (electrical)[160] or in a change in the intensity or wavelength of the fluorescence emission (optical).[121] Depending on the type of application, both electrical or optical signal transmission can be advantageous.[185] For sensitive measurement of electronic changes, field-effect transistors (FET) are often used in which the flow of charges within the SWCNTs is measured. The FET structures allow easy on-chip integration and can be parallelized to detect multiple target analytes simultaneously.[173] However, such sensors are more invasive for in vivo applications, as the entire device has to be inserted into the body. Optical detection with semiconducting SWCNTs is based on the radiative recombination of excitons in the near-infrared (NIR) by prior optical (fluorescence[186]) or electrical excitation (electroluminescence[187][188]). The emission in the NIR enables detection in the biological transparency window, where optical sensor applications benefit from reduced scattering and autofluorescence of biological samples and consequently a high signal-to-noise ratio.[189] Compared to optical sensors in the UV or visible range, the penetration depth in biological tissue is also increased. In addition to the advantage of a contactless readout SWCNTs have excellent photostability,[190] which enables long-term sensor applications. Furthermore, the nanoscale size of SWCNTs allows dense coating of surfaces which enables chemical imaging, e.g. of cellular release processes with high spatial and temporal resolution.[163][124] Detection of several target analytes is possible by the spatial arrangement of different SWCNT sensors in arrays[184][191][192] or by hyperspectral detection[184][193] based on monochiral SWCNT sensors that emit at different emission wavelengths. For fluorescence applications, however, optical filters to distinguish between excitation and emission and a NIR-sensitive detector must be used. Standard silicon detectors can also be used if monochiral SWCNTs (extractable by special purification processes) emitting closer to the visible range (800 – 900 nm) are used.[124][194] In order to avoid susceptibility of optical sensors to fluctuating ambient light, internal references such as SWCNTs that are modified to be non-responsive or stable NIR emitters[184][195] can be used. An alternative is to measure fluorescence lifetimes[196] instead of fluorescence intensities. Overall, SWCNTs therefore have great potential as building blocks for various biosensors. To render SWCNTs suitable for biosensing, their surface needs to be modified to ensure colloidal stability and provide a handle for biological recognition. Therefore, biosensing and surface modifications (functionalization) are closely related.[121][197][198]
Potential future applications include biomedical and environmental applications such as monitoring plant health in agriculture,[164][165][199] standoff process control in bioreactors, research/diagnostics of neuronal communication[200] and numerous diseases such as coagulation disorders,[201] diabetes,[179][202] cancer,[203] microbial and viral infections,[184][204] testing the efficacy of pharmaceuticals[205] or infection monitoring using smart implants. In industry, SWCNTs are already used as sensors in the detection of gases and odors in the form of an electronic nose[206] or in enzyme screening.[207]
Other current applications
[edit]- Easton-Bell Sports, Inc. have been in partnership with Zyvex Performance Materials, using CNT technology in a number of their bicycle components – including flat and riser handlebars, cranks, forks, seatposts, stems and aero bars.
- Amroy Europe Oy manufactures Hybtonite carbon nano-epoxy resins where carbon nanotubes have been chemically activated to bond to epoxy, resulting in a composite material that is 20% to 30% stronger than other composite materials. It has been used for wind turbines, marine paints and a variety of sports gear such as skis, ice hockey sticks, baseball bats, hunting arrows, and surfboards.[208]
- Surrey NanoSystems synthesizes carbon nanotubes to create vantablack ultra-absorptive black paint.
- "Gecko tape" (also called "nano tape") is often commercially sold as double-sided adhesive tape. It can be used to hang lightweight items such as pictures and decorative items on smooth walls without punching holes in the wall. The carbon nanotube arrays comprising the synthetic setae leave no residue after removal and can stay sticky in extreme temperatures.[209]
- Tips for atomic force microscope probes.[210]
Applications under development
[edit]Applications of nanotubes in development in academia and industry include:
- Medical devices: Using single wall carbon nanotubes in medical devices results in no skin contamination, high flexibility, and softness, which are crucial for healthcare applications.[211]
- Wearable electronics and 5G/6G communication: Electrodes with single wall carbon nanotubes (SWCNTs) exhibit excellent electrochemical properties and flexibility.[212][213]
- Bitumen and asphalt: The world's first test section of road pavement with single wall carbon nanotubes (SWCNTs) showed a 67% increase in resistance to cracks and ruts, increasing the lifespan of the materials.[214]
- Nanocomposites for aviation, automotive, and renewable energy markets: Modifying resin with just 0.02% single wall carbon nanotubes (SWCNTs) increases electrical conductivity by 276% without compromising the mechanical properties of fiber-reinforced polymers, also improving flexural properties and delaying thermal degradation.[215]
- Additive manufacturing: single wall carbon nanotubes (SWCNTs) are mixed with a suitable printing medium or used as a filler material in the printing process, creating complex structures with enhanced mechanical and electrical properties.[216]
- Utilizing carbon nanotubes as the channel material of carbon nanotube field-effect transistors.[217]
- Using carbon nanotubes as a scaffold for diverse microfabrication techniques.[218]
- Energy dissipation in self-organized nanostructures under the influence of an electric field.[219]
- Using carbon nanotubes for environmental monitoring due to their active surface area and their ability to absorb gases.[220]
- Jack Andraka used carbon nanotubes in his pancreatic cancer test. His method of testing won the Intel International Science and Engineering Fair Gordon E. Moore Award in the spring of 2012.[221]
- The Boeing Company has patented the use of carbon nanotubes for structural health monitoring[222] of composites used in aircraft structures. This technology is hoped to greatly reduce the risk of an in-flight failure caused by structural degradation of aircraft.
- Zyvex Technologies has also built a 54' maritime vessel, the Piranha Unmanned Surface Vessel, as a technology demonstrator for what is possible using CNT technology. CNTs help improve the structural performance of the vessel, resulting in a lightweight 8,000 lb boat that can carry a payload of 15,000 lb over a range of 2,500 miles.[223]
- IMEC is using carbon nanotubes for pellicles in semiconductor lithography.[224]
- In tissue engineering, carbon nanotubes have been used as scaffolding for bone growth.[225]
Carbon nanotubes can serve as additives to various structural materials. For instance, nanotubes form a tiny portion of the material(s) in some (primarily carbon fiber) baseball bats, golf clubs, car parts, or damascus steel.[226][227]
IBM expected carbon nanotube transistors to be used on Integrated Circuits by 2020.[228]
SWCNTs have found use in long lasting, faster charged lithium ion batteries;[229] polyamide car parts for e-painting;[230] automotive primers for cost benefits and better aesthetics of topcoats;[231] ESD floors;[232][233] electrically conductive lining coatings for tanks and pipes;[234] rubber parts with improved heat and oil aging stability;[235][236] conductive gelcoats for ATEX requirements and tooling conductive gelcoats for increased safety and efficiency;[237] and heating fiber coatings for infrastructure elements.[238]
Potential/Future applications
[edit]The strength and flexibility of carbon nanotubes makes them of potential use in controlling other nanoscale structures, which suggests they will have an important role in nanotechnology engineering.[239] The highest tensile strength of an individual multi-walled carbon nanotube has been tested to be 63 GPa.[56] Carbon nanotubes were found in Damascus steel from the 17th century, possibly helping to account for the legendary strength of the swords made of it.[240][241] Recently, several studies have highlighted the prospect of using carbon nanotubes as building blocks to fabricate three-dimensional macroscopic (>1mm in all three dimensions) all-carbon devices. Lalwani et al. have reported a novel radical initiated thermal crosslinking method to fabricated macroscopic, free-standing, porous, all-carbon scaffolds using single- and multi-walled carbon nanotubes as building blocks.[43] These scaffolds possess macro-, micro-, and nano- structured pores and the porosity can be tailored for specific applications. These 3D all-carbon scaffolds/architectures may be used for the fabrication of the next generation of energy storage, supercapacitors, field emission transistors, high-performance catalysis,[242] photovoltaics, and biomedical devices and implants.
CNTs are potential candidates for future via and wire material in nano-scale VLSI circuits. Eliminating electromigration reliability concerns that plague today's Cu interconnects, isolated (single and multi-wall) CNTs can carry current densities in excess of 1000 MA/cm2 without electromigration damage.[243]
Single-walled nanotubes are likely candidates for miniaturizing electronics. The most basic building block of these systems is an electric wire, and SWNTs with diameters of an order of a nanometre can be excellent conductors.[12][244] One useful application of SWNTs is in the development of the first intermolecular field-effect transistors (FET). The first intermolecular logic gate using SWCNT FETs was made in 2001.[245] A logic gate requires both a p-FET and an n-FET. Because SWNTs are p-FETs when exposed to oxygen and n-FETs otherwise, it is possible to expose half of an SWNT to oxygen and protect the other half from it. The resulting SWNT acts as a not logic gate with both p- and n-type FETs in the same molecule.
Large quantities of pure CNTs can be made into a freestanding sheet or film by surface-engineered tape-casting (SETC) fabrication technique which is a scalable method to fabricate flexible and foldable sheets with superior properties.[246][247] Another reported form factor is CNT fiber (a.k.a. filament) by wet spinning.[248] The fiber is either directly spun from the synthesis pot or spun from pre-made dissolved CNTs. Individual fibers can be turned into a yarn. Apart from its strength and flexibility, the main advantage is making an electrically conducting yarn. The electronic properties of individual CNT fibers (i.e. bundle of individual CNT) are governed by the two-dimensional structure of CNTs. The fibers were measured to have a resistivity only one order of magnitude higher than metallic conductors at 300 K (27 °C; 80 °F). By further optimizing the CNTs and CNT fibers, CNT fibers with improved electrical properties could be developed.[243][249]
CNT-based yarns are suitable for applications in energy and electrochemical water treatment when coated with an ion-exchange membrane.[250] Also, CNT-based yarns could replace copper as a winding material. Pyrhönen et al. (2015) have built a motor using CNT winding.[251][252]
See also
[edit]- Buckypaper
- Carbide-derived carbon
- Carbon nanocone
- Carbon nanofibers
- Carbon nanoscrolls
- Carbon nanotube computer
- Carbon nanotubes in photovoltaics
- Colossal carbon tube
- Diamond nanothread
- Filamentous carbon
- Molecular modelling
- Nanoflower
- Nano-I-beam
- Ninithi (nanotube modelling software)
- Optical properties of carbon nanotubes
- Organic semiconductor
References
[edit]This article incorporates public domain text from the National Institute of Environmental Health Sciences (NIEHS) as quoted.
- ^ a b c Pacios Pujadó M (2012). Carbon Nanotubes as Platforms for Biosensors with Electrochemical and Electronic Transduction (Thesis). Springer Theses. Springer Heidelberg. pp. xx, 208. doi:10.1007/978-3-642-31421-6. hdl:10803/84001. ISBN 978-3-642-31421-6. S2CID 199491391.
- ^ a b Monthioux M, Kuznetsov VL (August 2006). "Who should be given the credit for the discovery of carbon nanotubes?" (PDF). Carbon. 44 (9): 1621–1623. Bibcode:2006Carbo..44.1621M. doi:10.1016/j.carbon.2006.03.019. Archived (PDF) from the original on 9 October 2022.
- ^ Radushkevich LV (1952). О Структуре Углерода, Образующегося При Термическом Разложении Окиси Углерода На Железном Контакте [On the Structure of Carbon Formed During the Thermal Decomposition of Carbon Oxide on an Iron Contact] (PDF). Журнал Физической Химии [Journal of Physical Chemistry] (in Russian). 26: 88–95. Archived from the original (PDF) on 5 March 2016. Retrieved 5 April 2012.
- ^ Oberlin A, Endo M, Koyama T (March 1976). "Filamentous growth of carbon through benzene decomposition". Journal of Crystal Growth. 32 (3): 335–349. Bibcode:1976JCrGr..32..335O. doi:10.1016/0022-0248(76)90115-9.
- ^ a b c d e Eklund PC (2007). WTEC Panel Report on 'International Assessment of Research and Development of Carbon Nanotube Manufacturing and Applications' Final Report (PDF) (Report). World Technology Evaluation Center (WTEC). Archived from the original (PDF) on 11 March 2017. Retrieved 5 August 2015.
- ^ Oberlin A, Endo M, Koyama T (March 1976). "Filamentous growth of carbon through benzene decomposition" (PDF). Journal of Crystal Growth. 32 (3): 335–349. Bibcode:1976JCrGr..32..335O. doi:10.1016/0022-0248(76)90115-9. Archived (PDF) from the original on 9 October 2022.
- ^ JP 1982-58,966, Koyama T, Endo MT, "Method for Manufacturing Carbon Fibers by a Vapor Phase Process", issued 1983
- ^ Abrahamson J, Wiles PG, Rhoades BL (January 1999). "Structure of carbon fibres found on carbon arc anodes". Carbon. 37 (11): 1873–1874. Bibcode:1999Carbo..37.1873A. doi:10.1016/S0008-6223(99)00199-2.
- ^ Missing (1982). "Missing". Izvestiya Akademii Nauk SSSR Metally [Proceedings of the Academy of Sciences of the USSR. Metals] (in Russian). 3: 12–17. [full citation needed]
- ^ US 4663230, Tennent HG, "Carbon fibrils, method for producing same and compositions containing same", issued 1987-05-05
- ^ Iijima S (7 November 1991). "Helical microtubules of graphitic carbon". Nature. 354 (6348): 56–58. Bibcode:1991Natur.354...56I. doi:10.1038/354056a0. S2CID 4302490.
- ^ a b Mintmire JW, Dunlap BI, White CT (February 1992). "Are fullerene tubules metallic?". Physical Review Letters. 68 (5): 631–634. Bibcode:1992PhRvL..68..631M. doi:10.1103/PhysRevLett.68.631. PMID 10045950.
- ^ Iijima S, Ichihashi T (17 June 1993). "Single-shell carbon nanotubes of 1-nm diameter". Nature. 363 (6430): 603–605. Bibcode:1993Natur.363..603I. doi:10.1038/363603a0. S2CID 4314177.
- ^ Bethune DS, Kiang CH, De Vries MS, Gorman G, Savoy R, Vazquez J, et al. (17 June 1993). "Cobalt-catalyzed growth of carbon nanotubes with single-atomic-layer walls". Nature. 363 (6430): 605–607. Bibcode:1993Natur.363..605B. doi:10.1038/363605a0. S2CID 4321984.
- ^ Thess A, Lee R, Nikolaev P, Dai H, Petit P, Robert J, et al. (July 1996). "Crystalline Ropes of Metallic Carbon Nanotubes". Science. 273 (5274): 483–487. Bibcode:1996Sci...273..483T. doi:10.1126/science.273.5274.483. PMID 8662534. S2CID 13284203.
- ^ Krätschmer W, Lamb LD, Fostiropoulos KH, Huffman DR (1990). "Solid C60: a new form of carbon". Nature. 347 (6291): 354–358. Bibcode:1990Natur.347..354K. doi:10.1038/347354a0. S2CID 4359360.
- ^ Kokarneswaran M, Selvaraj P, Ashokan T, Perumal S, Sellappan P, Murugan KD, et al. (November 2020). "Discovery of carbon nanotubes in sixth century BC potteries from Keeladi, India". Scientific Reports. 10 (1): 19786. Bibcode:2020NatSR..1019786K. doi:10.1038/s41598-020-76720-z. PMC 7666134. PMID 33188244.
- ^ a b Sinnott SB, Andrews R (July 2001). "Carbon Nanotubes: Synthesis, Properties, and Applications". Critical Reviews in Solid State and Materials Sciences. 26 (3): 145–249. Bibcode:2001CRSSM..26..145S. doi:10.1080/20014091104189. S2CID 95444574.
- ^ Zhao X, Liu Y, Inoue S, Suzuki T, Jones RO, Ando Y (March 2004). "Smallest carbon nanotube is 3 a in diameter" (PDF). Physical Review Letters. 92 (12): 125502. Bibcode:2004PhRvL..92l5502Z. doi:10.1103/PhysRevLett.92.125502. PMID 15089683. Archived (PDF) from the original on 9 October 2022.
- ^ Torres-Dias AC (2017). "From mesoscale to nanoscale mechanics in single-wall carbon nanotubes". Carbon. 123: 145–150. Bibcode:2017Carbo.123..145T. doi:10.1016/j.carbon.2017.07.036.
- ^ Hayashi T, Kim YA, Matoba T, Esaka M, Nishimura K, Tsukada T, et al. (2003). "Smallest Freestanding Single-Walled Carbon Nanotube". Nano Letters. 3 (7): 887–889. Bibcode:2003NanoL...3..887H. doi:10.1021/nl034080r.
- ^ Guan L, Suenaga K, Iijima S (February 2008). "Smallest carbon nanotube assigned with atomic resolution accuracy". Nano Letters. 8 (2): 459–462. Bibcode:2008NanoL...8..459G. doi:10.1021/nl072396j. PMID 18186659.
- ^ Zhang R, Zhang Y, Zhang Q, Xie H, Qian W, Wei F (July 2013). "Growth of half-meter long carbon nanotubes based on Schulz-Flory distribution". ACS Nano. 7 (7): 6156–6161. doi:10.1021/nn401995z. PMID 23806050.
- ^ Wang X, Li Q, Xie J, Jin Z, Wang J, Li Y, et al. (September 2009). "Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates". Nano Letters. 9 (9): 3137–3141. Bibcode:2009NanoL...9.3137W. CiteSeerX 10.1.1.454.2744. doi:10.1021/nl901260b. PMID 19650638.
- ^ Jasti R, Bhattacharjee J, Neaton JB, Bertozzi CR (December 2008). "Synthesis, characterization, and theory of [9]-, [12]-, and [18]cycloparaphenylene: carbon nanohoop structures". Journal of the American Chemical Society. 130 (52): 17646–17647. doi:10.1021/ja807126u. PMC 2709987. PMID 19055403.
- ^ Cheung KY, Segawa Y, Itami K (November 2020). "Synthetic Strategies of Carbon Nanobelts and Related Belt-Shaped Polycyclic Aromatic Hydrocarbons". Chemistry: A European Journal. 26 (65): 14791–14801. doi:10.1002/chem.202002316. PMID 32572996. S2CID 219983922.
- ^ "Densest array of carbon nanotubes grown to date". KurzweilAI. 27 September 2013.
- ^ Sugime H, Esconjauregui S, Yang J, D'Arsié L, Oliver RA, Bhardwaj S, et al. (12 August 2013). "Low temperature growth of ultra-high mass density carbon nanotube forests on conductive supports". Applied Physics Letters. 103 (7): 073116. Bibcode:2013ApPhL.103g3116S. doi:10.1063/1.4818619.
- ^ "Nanotechnologies — Vocabulary — Part 3: Carbon nano-objects". www.iso.org. Retrieved 22 October 2024.
- ^ Hamada N, Sawada SI, Oshiyama A (March 1992). "New one-dimensional conductors: Graphitic microtubules". Physical Review Letters. 68 (10): 1579–1581. Bibcode:1992PhRvL..68.1579H. doi:10.1103/PhysRevLett.68.1579. PMID 10045167.
- ^ Wilder JW, Venema LC, Rinzler AG, Smalley RE, Dekker C (1 January 1998). "Electronic structure of atomically resolved carbon nanotubes". Nature. 391 (6662): 59–62. Bibcode:1998Natur.391...59W. doi:10.1038/34139. S2CID 205003208.
- ^ Das S (March 2013). "A review on Carbon nano-tubes – A new era of nanotechnology" (PDF). International Journal of Emerging Technology and Advanced Engineering. 3 (3): 774–781. CiteSeerX 10.1.1.413.7576. Archived (PDF) from the original on 9 October 2022.
- ^ Piao Y, Chen CF, Green AA, Kwon H, Hersam MC, Lee CS, et al. (7 July 2011). "Optical and Electrical Properties of Inner Tubes in Outer Wall-Selectively Functionalized Double-Wall Carbon Nanotubes". The Journal of Physical Chemistry Letters. 2 (13): 1577–1582. doi:10.1021/jz200687u.
- ^ Flahaut E, Bacsa R, Peigney A, Laurent C (June 2003). "Gram-scale CCVD synthesis of double-walled carbon nanotubes" (PDF). Chemical Communications (12): 1442–1443. doi:10.1039/b301514a. PMID 12841282. S2CID 30627446. Archived (PDF) from the original on 9 October 2022.
- ^ Cumings J, Zettl A (July 2000). "Low-friction nanoscale linear bearing realized from multiwall carbon nanotubes". Science. 289 (5479): 602–604. Bibcode:2000Sci...289..602C. CiteSeerX 10.1.1.859.7671. doi:10.1126/science.289.5479.602. PMID 10915618.
- ^ Zavalniuk V, Marchenko S (2011). "Theoretical analysis of telescopic oscillations in multi-walled carbon nanotubes" (PDF). Low Temperature Physics. 37 (4): 337–342. arXiv:0903.2461. Bibcode:2011LTP....37..337Z. doi:10.1063/1.3592692. S2CID 51932307. Archived (PDF) from the original on 9 October 2022.
- ^ Chernozatonskii LA (1992). "Carbon nanotube connectors and planar jungle gyms". Physics Letters A. 172 (3): 173–176. Bibcode:1992PhLA..172..173C. doi:10.1016/0375-9601(92)90978-u.
- ^ Menon M, Srivastava D (1 December 1997). "Carbon Nanotube 'T Junctions': Nanoscale Metal-Semiconductor-Metal Contact Devices". Physical Review Letters. 79 (22): 4453–4456. Bibcode:1997PhRvL..79.4453M. doi:10.1103/physrevlett.79.4453.
- ^ Lambin P (1996). "Atomic structure and electronic properties of bent carbon nanotubes". Synth. Met. 77 (1–3): 249–1254. doi:10.1016/0379-6779(96)80097-x.
- ^ Ma KL (2011). "Electronic transport properties of junctions between carbon nanotubes and graphene nanoribbons". European Physical Journal B. 83 (4): 487–492. Bibcode:2011EPJB...83..487M. doi:10.1140/epjb/e2011-20313-9. S2CID 119497542.
- ^ Harris PJ, Suarez-Martinez I, Marks NA (December 2016). "The structure of junctions between carbon nanotubes and graphene shells" (PDF). Nanoscale. 8 (45): 18849–18854. doi:10.1039/c6nr06461b. PMID 27808332. S2CID 42241359. Archived (PDF) from the original on 9 October 2022.
- ^ Dimitrakakis GK, Tylianakis E, Froudakis GE (October 2008). "Pillared graphene: a new 3-D network nanostructure for enhanced hydrogen storage". Nano Letters. 8 (10): 3166–3170. Bibcode:2008NanoL...8.3166D. doi:10.1021/nl801417w. PMID 18800853.
- ^ a b Lalwani G, Kwaczala AT, Kanakia S, Patel SC, Judex S, Sitharaman B (March 2013). "Fabrication and Characterization of Three-Dimensional Macroscopic All-Carbon Scaffolds". Carbon. 53: 90–100. doi:10.1016/j.carbon.2012.10.035. PMC 3578711. PMID 23436939.
- ^ Lalwani G, Gopalan A, D'Agati M, Sankaran JS, Judex S, Qin YX, et al. (October 2015). "Porous three-dimensional carbon nanotube scaffolds for tissue engineering". Journal of Biomedical Materials Research. Part A. 103 (10): 3212–3225. doi:10.1002/jbm.a.35449. PMC 4552611. PMID 25788440.
- ^ Noyce SG, Vanfleet RR, Craighead HG, Davis RC (March 2019). "High surface-area carbon microcantilevers". Nanoscale Advances. 1 (3): 1148–1154. Bibcode:2019NanoA...1.1148N. doi:10.1039/C8NA00101D. PMC 9418787. PMID 36133213.
- ^ Nasibulin AG, Pikhitsa PV, Jiang H, Brown DP, Krasheninnikov AV, Anisimov AS, et al. (March 2007). "A novel hybrid carbon material". Nature Nanotechnology. 2 (3): 156–161. Bibcode:2007NatNa...2..156N. doi:10.1038/nnano.2007.37. PMID 18654245.
- ^ Smith BW, Monthioux M, Luzzi DE (1998). "Encapsulated C-60 in carbon nanotubes". Nature. 396 (6709): 323–324. Bibcode:1998Natur.396R.323S. doi:10.1038/24521. S2CID 30670931.
- ^ Smith BW, Luzzi DE (2000). "Formation mechanism of fullerene peapods and coaxial tubes: a path to large scale synthesis". Chem. Phys. Lett. 321 (1–2): 169–174. Bibcode:2000CPL...321..169S. doi:10.1016/S0009-2614(00)00307-9.
- ^ Su H, Goddard WA, Zhao Y (2006). "Dynamic friction force in a carbon peapod oscillator" (PDF). Nanotechnology. 17 (22): 5691–5695. arXiv:cond-mat/0611671. Bibcode:2006Nanot..17.5691S. doi:10.1088/0957-4484/17/22/026. S2CID 18165997. Archived (PDF) from the original on 9 October 2022.
- ^ Wang M, Li CM (January 2010). "An oscillator in a carbon peapod controllable by an external electric field: a molecular dynamics study". Nanotechnology. 21 (3): 035704. Bibcode:2010Nanot..21c5704W. doi:10.1088/0957-4484/21/3/035704. PMID 19966399. S2CID 12358310.
- ^ a b Liu L, Guo GY, Jayanthi CS, Wu SY (May 2002). "Colossal paramagnetic moments in metallic carbon nanotori". Physical Review Letters. 88 (21): 217206. Bibcode:2002PhRvL..88u7206L. doi:10.1103/PhysRevLett.88.217206. PMID 12059501.
- ^ Huhtala M, Kuronen A, Kaski K (2002). "Carbon nanotube structures: Molecular dynamics simulation at realistic limit" (PDF). Computer Physics Communications. 146 (1): 30–37. Bibcode:2002CoPhC.146...30H. doi:10.1016/S0010-4655(02)00432-0. Archived from the original (PDF) on 27 June 2008.
- ^ Parker CB, Raut AS, Brown B, Stoner BR, Glass JT (2012). "Three-dimensional arrays of graphenated carbon nanotubes". J. Mater. Res. 7. 27 (7): 1046–1053. Bibcode:2012JMatR..27.1046P. doi:10.1557/jmr.2012.43. S2CID 137964473.
- ^ Stoner BR, Glass JT (2012). "Carbon nanostructures: a morphological classification for charge density optimization". Diamond and Related Materials. 23: 130–134. Bibcode:2012DRM....23..130S. doi:10.1016/j.diamond.2012.01.034.
- ^ Liu Q, Ren W, Chen ZG, Yin L, Li F, Cong H, et al. (2009). "Semiconducting properties of cup-stacked carbon nanotubes" (PDF). Carbon. 47 (3): 731–736. Bibcode:2009Carbo..47..731L. doi:10.1016/j.carbon.2008.11.005. Archived from the original (PDF) on 9 January 2015.
- ^ a b Yu MF, Lourie O, Dyer MJ, Moloni K, Kelly TF, Ruoff RS (January 2000). "Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load". Science. 287 (5453): 637–640. Bibcode:2000Sci...287..637Y. doi:10.1126/science.287.5453.637. PMID 10649994. S2CID 10758240.
- ^ a b Peng B, Locascio M, Zapol P, Li S, Mielke SL, Schatz GC, et al. (October 2008). "Measurements of near-ultimate strength for multiwalled carbon nanotubes and irradiation-induced crosslinking improvements". Nature Nanotechnology. 3 (10): 626–631. doi:10.1038/nnano.2008.211. PMID 18839003.
- ^ Collins PG, Avouris P (December 2000). "Nanotubes for electronics". Scientific American. 283 (6): 62–69. Bibcode:2000SciAm.283f..62C. doi:10.1038/scientificamerican1200-62. PMID 11103460.
- ^ a b Filleter T, Bernal R, Li S, Espinosa HD (July 2011). "Ultrahigh strength and stiffness in cross-linked hierarchical carbon nanotube bundles". Advanced Materials. 23 (25): 2855–2860. Bibcode:2011AdM....23.2855F. doi:10.1002/adma.201100547. PMID 21538593. S2CID 6363504.
- ^ Jensen K, Mickelson W, Kis A, Zettl A (26 November 2007). "Buckling and kinking force measurements on individual multiwalled carbon nanotubes". Physical Review B. 76 (19): 195436. Bibcode:2007PhRvB..76s5436J. doi:10.1103/PhysRevB.76.195436.
- ^ Treacy MM, Ebbesen TW, Gibson JM (June 1996). "Exceptionally high Young's modulus observed for individual carbon nanotubes". Nature. 381 (6584): 678–680. Bibcode:1996Natur.381..678T. doi:10.1038/381678a0.
- ^ Tans SJ, Devoret MH, Dai H, Thess A, Smalley RE, Geerligs LJ, et al. (April 1997). "Individual single-wall carbon nanotubes as quantum wires". Nature. 386 (6624): 474–477. Bibcode:1997Natur.386..474T. doi:10.1038/386474a0. S2CID 4366705.
- ^ Laird EA, Kuemmeth F, Steele GA, Grove-Rasmussen K, Nygård J, Flensberg K, et al. (2015). "Quantum Transport in Carbon Nanotubes". Reviews of Modern Physics. 87 (3): 703–764. arXiv:1403.6113. Bibcode:2015RvMP...87..703L. doi:10.1103/RevModPhys.87.703. S2CID 119208985.
- ^ a b Lu X, Chen Z (October 2005). "Curved pi-conjugation, aromaticity, and the related chemistry of small fullerenes (< C60) and single-walled carbon nanotubes". Chemical Reviews. 105 (10): 3643–3696. doi:10.1021/cr030093d. PMID 16218563.
- ^ Hong S, Myung S (April 2007). "Nanotube electronics: a flexible approach to mobility". Nature Nanotechnology. 2 (4): 207–208. Bibcode:2007NatNa...2..207H. doi:10.1038/nnano.2007.89. PMID 18654263.
- ^ Vasylenko A, Wynn J, Medeiros PV, Morris AJ, Sloan J, Quigley D (2017). "Encapsulated nanowires: Boosting electronic transport in carbon nanotubes". Physical Review B. 95 (12): 121408. arXiv:1611.04867. Bibcode:2017PhRvB..95l1408V. doi:10.1103/PhysRevB.95.121408. S2CID 59023024.
- ^ Charlier JC, Blase X, Roche S (2007). "Electronic and transport properties of nanotubes" (PDF). Reviews of Modern Physics. 79 (2): 677–732. Bibcode:2007RvMP...79..677C. doi:10.1103/RevModPhys.79.677.
- ^ Tang ZK, Zhang L, Wang N, Zhang XX, Wen GH, Li GD, et al. (June 2001). "Superconductivity in 4 angstrom single-walled carbon nanotubes". Science. 292 (5526): 2462–2465. Bibcode:2001Sci...292.2462T. doi:10.1126/science.1060470. PMID 11431560. S2CID 44987798.
- ^ Takesue I, Haruyama J, Kobayashi N, Chiashi S, Maruyama S, Sugai T, et al. (February 2006). "Superconductivity in entirely end-bonded multiwalled carbon nanotubes". Physical Review Letters. 96 (5): 057001. arXiv:cond-mat/0509466. Bibcode:2006PhRvL..96e7001T. doi:10.1103/PhysRevLett.96.057001. PMID 16486971. S2CID 119049151.
- ^ Lortz R, Zhang Q, Shi W, Ye JT, Ye JT, Qiu C, et al. (May 2009). "Superconducting characteristics of 4-A carbon nanotube-zeolite composite". Proceedings of the National Academy of Sciences of the United States of America. 106 (18): 7299–7303. doi:10.1073/pnas.0813162106. PMC 2678622. PMID 19369206.
- ^ Bockrath M (1 March 2006). "The weakest link". Nature Physics. 2 (3): 155–156. doi:10.1038/nphys252. S2CID 125902065.
- ^ Liu AT, Kunai Y, Cottrill AL, Kaplan A, Zhang G, Kim H, et al. (June 2021). "Solvent-induced electrochemistry at an electrically asymmetric carbon Janus particle". Nature Communications. 12 (1): 3415. Bibcode:2021NatCo..12.3415L. doi:10.1038/s41467-021-23038-7. PMC 8184849. PMID 34099639. S2CID 235370395.
- ^ a b Trafton A (7 June 2021). "MIT Engineers Have Discovered a Completely New Way of Generating Electricity". SciTechDaily. Retrieved 8 June 2021.
- ^ Carbon-Based Magnetism: An Overview of the Magnetism of Metal Free Carbon-based Compounds and Materials, Tatiana Makarova and Fernando Palacio (eds.), Elsevier, 2006
- ^ Yang L, Anantram MP, Han J, Lu JP (15 November 1999). "Band-gap change of carbon nanotubes: Effect of small uniaxial and torsional strain". Physical Review B. 60 (19): 13874–13878. arXiv:cond-mat/9811263. Bibcode:1999PhRvB..6013874Y. doi:10.1103/PhysRevB.60.13874. ISSN 0163-1829.
- ^ Obitayo W, Liu T (2012). "A Review: Carbon Nanotube-Based Piezoresistive Strain Sensors". Journal of Sensors. 2012: 1–15. doi:10.1155/2012/652438. ISSN 1687-725X.
- ^ Misewich JA, Martel R, Avouris P, Tsang JC, Heinze S, Tersoff J (May 2003). "Electrically induced optical emission from a carbon nanotube FET". Science. 300 (5620): 783–786. Bibcode:2003Sci...300..783M. doi:10.1126/science.1081294. PMID 12730598. S2CID 36336745.
- ^ Chen J, Perebeinos V, Freitag M, Tsang J, Fu Q, Liu J, et al. (November 2005). "Bright infrared emission from electrically induced excitons in carbon nanotubes". Science. 310 (5751): 1171–1174. Bibcode:2005Sci...310.1171C. doi:10.1126/science.1119177. PMID 16293757. S2CID 21960183.
- ^ Freitag M, Martin Y, Misewich JA, Martel R, Avouris P (2003). "Photoconductivity of Single Carbon Nanotubes". Nano Letters. 3 (8): 1067–1071. Bibcode:2003NanoL...3.1067F. doi:10.1021/nl034313e.
- ^ Itkis ME, Borondics F, Yu A, Haddon RC (April 2006). "Bolometric infrared photoresponse of suspended single-walled carbon nanotube films". Science. 312 (5772): 413–416. Bibcode:2006Sci...312..413I. doi:10.1126/science.1125695. PMID 16627739.
- ^ Star A, Lu Y, Bradley K, Grüner G (2004). "Nanotube Optoelectronic Memory Devices". Nano Letters. 4 (9): 1587–1591. Bibcode:2004NanoL...4.1587S. doi:10.1021/nl049337f.
- ^ Paul Cherukuri, Sergei M. Bachilo, Silvio H. Litovsky, R. Bruce Weisman (2004). "Near-Infrared Fluorescence Microscopy of Single-Walled Carbon Nanotubes in Phagocytic Cells". Journal of the American Chemical Society. 126 (48): 15638–15639. doi:10.1021/ja0466311. PMID 15571374.
- ^ Kevin Welsher, Sarah P. Sherlock, Hongjie Dai (2011). "Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window". Proceedings of the National Academy of Sciences. 108 (22): 8943–8948. arXiv:1105.3536. Bibcode:2011PNAS..108.8943W. doi:10.1073/pnas.1014501108. PMC 3107273. PMID 21576494.
- ^ Paul W. Barone, Seunghyun Baik, Daniel A. Heller, Michael S. Strano (2005). "Near-infrared optical sensors based on single-walled carbon nanotubes". Nature Materials. 4 (1): 86–92. Bibcode:2005NatMa...4...86B. doi:10.1038/nmat1276. PMID 15592477. S2CID 43558342.
- ^ Berber S, Kwon YK, Tomanek D (May 2000). "Unusually high thermal conductivity of carbon nanotubes". Physical Review Letters. 84 (20): 4613–4616. arXiv:cond-mat/0002414. Bibcode:2000PhRvL..84.4613B. doi:10.1103/PhysRevLett.84.4613. PMID 10990753. S2CID 9006722.
- ^ Kim P, Shi L, Majumdar A, McEuen PL (November 2001). "Thermal transport measurements of individual multiwalled nanotubes". Physical Review Letters. 87 (21): 215502. arXiv:cond-mat/0106578. Bibcode:2001PhRvL..87u5502K. doi:10.1103/PhysRevLett.87.215502. PMID 11736348. S2CID 12533685.
- ^ Pop E, Mann D, Wang Q, Goodson K, Dai H (January 2006). "Thermal conductance of an individual single-wall carbon nanotube above room temperature". Nano Letters. 6 (1): 96–100. arXiv:cond-mat/0512624. Bibcode:2006NanoL...6...96P. doi:10.1021/nl052145f. PMID 16402794. S2CID 14874373.
- ^ Sinha S, Barjami S, Iannacchione G, Schwab A, Muench G (5 June 2005). "Off-axis thermal properties of carbon nanotube films". Journal of Nanoparticle Research. 7 (6): 651–657. Bibcode:2005JNR.....7..651S. doi:10.1007/s11051-005-8382-9. S2CID 138479725.
- ^ Koziol KK, Janas D, Brown E, Hao L (1 April 2017). "Thermal properties of continuously spun carbon nanotube fibres". Physica E. 88: 104–108. Bibcode:2017PhyE...88..104K. doi:10.1016/j.physe.2016.12.011.
- ^ Kumanek B, Janas D (May 2019). "Thermal conductivity of carbon nanotube networks: a review". Journal of Materials Science. 54 (10): 7397–7427. Bibcode:2019JMatS..54.7397K. doi:10.1007/s10853-019-03368-0.
- ^ Thostenson E, Li C, Chou T (2005). "Nanocomposites in context". Composites Science and Technology. 65 (3–4): 491–51. doi:10.1016/j.compscitech.2004.11.003.
- ^ Mingo N, Stewart DA, Broido DA, Srivastava D (2008). "Phonon transmission through defects in carbon nanotubes from first principles". Phys. Rev. B. 77 (3): 033418. Bibcode:2008PhRvB..77c3418M. doi:10.1103/PhysRevB.77.033418. hdl:1813/10898.
- ^ The Effectiveness Mechanisms of Carbon Nanotubes (CNTs) as Reinforcements for Magnesium-Based Composites for Biomedical Applications: A Review Nanomaterials 2024, 14, 756. https://doi.org/10.3390/nano14090756

- ^ Endo M (October 2004). "Applications of carbon nanotubes in the twenty-first century". Philosophical Transactions of the Royal Society of London. Series A: Mathematical, Physical and Engineering Sciences. 362 (1823): 2223–2238. doi:10.1098/rsta.2004.1437. PMID 15370479. S2CID 20752554.
- ^ Zhou Z (January 2003). "Producing cleaner double-walled carbon nanotubes in a floating catalyst system". Carbon. 41 (13): 2607–2611. Bibcode:2003Carbo..41.2607Z. doi:10.1016/S0008-6223(03)00336-1.
- ^ a b Nikolaev P (April 2004). "Gas-phase production of single-walled carbon nanotubes from carbon monoxide: a review of the hipco process". Journal of Nanoscience and Nanotechnology. 4 (4): 307–316. doi:10.1166/jnn.2004.066. PMID 15296221.
- ^ Schulz MJ, Shanov VN, Yun Y (2009). Nanomedicine Design of Particles, Sensors, Motors, Implants, Robots, and Devices. Artech House. ISBN 978-1-59693-280-7.
- ^ Takeuchi K, Hayashi T, Kim YA, Fujisawa K, Endo M (February 2014). "The state-of-the-art science and applications of carbon nanotubes". Nanosystems: Physics, Chemistry, Mathematics. 5 (1): 15–24.
- ^ Bronikowski MJ, Willis PA, Colbert DT, Smith KA, Smalley RE (July 2001). "Gas-phase production of carbon single-walled nanotubes from carbon monoxide via the HiPco process: A parametric study". Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films. 19 (4): 1800–1805. Bibcode:2001JVSTA..19.1800B. doi:10.1116/1.1380721. S2CID 3846517.
- ^ Itkis ME, Perea DE, Niyogi S, Rickard SM, Hamon MA, Hu H, et al. (1 March 2003). "Purity Evaluation of As-Prepared Single-Walled Carbon Nanotube Soot by Use of Solution-Phase Near-IR Spectroscopy". Nano Letters. 3 (3): 309–314. Bibcode:2003NanoL...3..309I. doi:10.1021/nl025926e.
- ^ Wang L, Pumera M (October 2014). "Residual metallic impurities within carbon nanotubes play a dominant role in supposedly "metal-free" oxygen reduction reactions". Chemical Communications. 50 (84): 12662–12664. doi:10.1039/C4CC03271C. PMID 25204561.
- ^ Eatemadi A, Daraee H, Karimkhanloo H, Kouhi M, Zarghami N, Akbarzadeh A, et al. (13 August 2014). "Carbon nanotubes: properties, synthesis, purification, and medical applications". Nanoscale Research Letters. 9 (1): 393. Bibcode:2014NRL.....9..393E. doi:10.1186/1556-276X-9-393. PMC 4141964. PMID 25170330.
- ^ Chen F, Wang B, Chen Y, Li LJ (1 October 2007). "Toward the Extraction of Single Species of Single-Walled Carbon Nanotubes Using Fluorene-Based Polymers". Nano Letters. 7 (10): 3013–3017. Bibcode:2007NanoL...7.3013C. doi:10.1021/nl071349o. ISSN 1530-6984. PMID 17867716.
- ^ Nish A, Hwang JY, Doig J, Nicholas RJ (October 2007). "Highly selective dispersion of single-walled carbon nanotubes using aromatic polymers". Nature Nanotechnology. 2 (10): 640–646. Bibcode:2007NatNa...2..640N. doi:10.1038/nnano.2007.290. ISSN 1748-3395. PMID 18654390.
- ^ Lemasson FA, Strunk T, Gerstel P, Hennrich F, Lebedkin S, Barner-Kowollik C, et al. (2 February 2011). "Selective Dispersion of Single-Walled Carbon Nanotubes with Specific Chiral Indices by Poly( N -decyl-2,7-carbazole)". Journal of the American Chemical Society. 133 (4): 652–655. doi:10.1021/ja105722u. ISSN 0002-7863. PMID 21171609. S2CID 23209007.
- ^ Arnold MS, Stupp SI, Hersam MC (1 April 2005). "Enrichment of Single-Walled Carbon Nanotubes by Diameter in Density Gradients". Nano Letters. 5 (4): 713–718. Bibcode:2005NanoL...5..713A. doi:10.1021/nl050133o. ISSN 1530-6984. PMID 15826114.
- ^ Green AA, Hersam MC (17 May 2011). "Nearly Single-Chirality Single-Walled Carbon Nanotubes Produced via Orthogonal Iterative Density Gradient Ultracentrifugation". Advanced Materials. 23 (19): 2185–2190. Bibcode:2011AdM....23.2185G. doi:10.1002/adma.201100034. ISSN 0935-9648. PMID 21472798. S2CID 5375678.
- ^ Flavel BS, Kappes MM, Krupke R, Hennrich F (23 April 2013). "Separation of Single-Walled Carbon Nanotubes by 1-Dodecanol-Mediated Size-Exclusion Chromatography". ACS Nano. 7 (4): 3557–3564. doi:10.1021/nn4004956. ISSN 1936-0851. PMID 23540203.
- ^ Huang X, Mclean RS, Zheng M (1 October 2005). "High-Resolution Length Sorting and Purification of DNA-Wrapped Carbon Nanotubes by Size-Exclusion Chromatography". Analytical Chemistry. 77 (19): 6225–6228. doi:10.1021/ac0508954. ISSN 0003-2700. PMID 16194082.
- ^ Moore KE, Pfohl M, Hennrich F, Chakradhanula VS, Kuebel C, Kappes MM, et al. (22 July 2014). "Separation of Double-Walled Carbon Nanotubes by Size Exclusion Column Chromatography". ACS Nano. 8 (7): 6756–6764. doi:10.1021/nn500756a. ISSN 1936-0851. PMID 24896840.
- ^ Ao G, Khripin CY, Zheng M (23 July 2014). "DNA-Controlled Partition of Carbon Nanotubes in Polymer Aqueous Two-Phase Systems". Journal of the American Chemical Society. 136 (29): 10383–10392. doi:10.1021/ja504078b. ISSN 0002-7863. PMID 24976036.
- ^ Fagan JA, Khripin CY, Silvera Batista CA, Simpson JR, Hároz EH, Hight Walker AR, et al. (May 2014). "Isolation of Specific Small-Diameter Single-Wall Carbon Nanotube Species via Aqueous Two-Phase Extraction". Advanced Materials. 26 (18): 2800–2804. Bibcode:2014AdM....26.2800F. doi:10.1002/adma.201304873. ISSN 0935-9648. PMID 24448916. S2CID 205253171.
- ^ Lyu M, Meany B, Yang J, Li Y, Zheng M (26 December 2019). "Toward Complete Resolution of DNA/Carbon Nanotube Hybrids by Aqueous Two-Phase Systems". Journal of the American Chemical Society. 141 (51): 20177–20186. doi:10.1021/jacs.9b09953. ISSN 0002-7863. PMID 31783712. S2CID 208498347.
- ^ Li H, Gordeev G, Garrity O, Reich S, Flavel BS (28 January 2019). "Separation of Small-Diameter Single-Walled Carbon Nanotubes in One to Three Steps with Aqueous Two-Phase Extraction". ACS Nano. 13 (2): 2567–2578. doi:10.1021/acsnano.8b09579. ISSN 1936-0851. PMID 30673278. S2CID 59224819.
- ^ Yang F, Wang M, Zhang D, Yang J, Zheng M, Li Y (11 March 2020). "Chirality Pure Carbon Nanotubes: Growth, Sorting, and Characterization". Chemical Reviews. 120 (5): 2693–2758. doi:10.1021/acs.chemrev.9b00835. ISSN 0009-2665. PMID 32039585. S2CID 211071215.
- ^ Janas D (21 December 2017). "Towards monochiral carbon nanotubes: a review of progress in the sorting of single-walled carbon nanotubes". Materials Chemistry Frontiers. 2 (1): 36–63. doi:10.1039/C7QM00427C. ISSN 2052-1537.
- ^ Wei X, Li S, Wang W, Zhang X, Zhou W, Xie S, et al. (May 2022). "Recent Advances in Structure Separation of Single-Wall Carbon Nanotubes and Their Application in Optics, Electronics, and Optoelectronics". Advanced Science. 9 (14): e2200054. doi:10.1002/advs.202200054. ISSN 2198-3844. PMC 9108629. PMID 35293698.
- ^ Zheng M, Semke ED (1 May 2007). "Enrichment of Single Chirality Carbon Nanotubes". Journal of the American Chemical Society. 129 (19): 6084–6085. doi:10.1021/ja071577k. ISSN 0002-7863. PMID 17458969.
- ^ Liu H, Nishide D, Tanaka T, Kataura H (10 May 2011). "Large-scale single-chirality separation of single-wall carbon nanotubes by simple gel chromatography". Nature Communications. 2 (1): 309. Bibcode:2011NatCo...2..309L. doi:10.1038/ncomms1313. ISSN 2041-1723. PMC 3113293. PMID 21556063.
- ^ Tanaka T, Jin H, Miyata Y, Fujii S, Suga H, Naitoh Y, et al. (8 April 2009). "Simple and Scalable Gel-Based Separation of Metallic and Semiconducting Carbon Nanotubes". Nano Letters. 9 (4): 1497–1500. Bibcode:2009NanoL...9.1497T. doi:10.1021/nl8034866. ISSN 1530-6984. PMID 19243112.
- ^ a b c d Ackermann J, Metternich JT, Herbertz S, Kruss S (25 April 2022). "Biosensing with Fluorescent Carbon Nanotubes". Angewandte Chemie International Edition. 61 (18): e202112372. doi:10.1002/anie.202112372. ISSN 1433-7851. PMC 9313876. PMID 34978752.
- ^ Nißler R, Kurth L, Li H, Spreinat A, Kuhlemann I, Flavel BS, et al. (27 April 2021). "Sensing with Chirality-Pure Near-Infrared Fluorescent Carbon Nanotubes". Analytical Chemistry. 93 (16): 6446–6455. doi:10.1021/acs.analchem.1c00168. ISSN 0003-2700. PMID 33830740.
- ^ Nißler R, Ackermann J, Ma C, Kruss S (19 July 2022). "Prospects of Fluorescent Single-Chirality Carbon Nanotube-Based Biosensors". Analytical Chemistry. 94 (28): 9941–9951. doi:10.1021/acs.analchem.2c01321. ISSN 0003-2700. PMID 35786856. S2CID 250283972.
- ^ a b c d Ackermann J, Stegemann J, Smola T, Reger E, Jung S, Schmitz A, et al. (April 2023). "High Sensitivity Near-Infrared Imaging of Fluorescent Nanosensors". Small. 19 (14): e2206856. doi:10.1002/smll.202206856. ISSN 1613-6810. PMID 36610045.
- ^ Nadeem A, Kindopp A, Wyllie I, Hubert L, Joubert J, Lucente S, et al. (26 July 2023). "Enhancing Intracellular Optical Performance and Stability of Engineered Nanomaterials via Aqueous Two-Phase Purification". Nano Letters. 23 (14): 6588–6595. Bibcode:2023NanoL..23.6588N. doi:10.1021/acs.nanolett.3c01727. ISSN 1530-6984. PMC 11068083. PMID 37410951. S2CID 259356687.
- ^ Bekyarova E, Davis M, Burch T, Itkis ME, Zhao B, Sunshine S, et al. (9 October 2004). "Chemically Functionalized Single-Walled Carbon Nanotubes as Ammonia Sensors" (PDF). J. Phys. Chem. B. 108 (51). Washington, D.C.: ACS Publications: 19717–19720. doi:10.1021/jp0471857. ISSN 1520-5207. S2CID 96173424.
- ^ Stando G, Łukawski D, Lisiecki F, Janas D (January 2019). "Intrinsic hydrophilic character of carbon nanotube networks". Applied Surface Science. 463: 227–233. Bibcode:2019ApSS..463..227S. doi:10.1016/j.apsusc.2018.08.206. S2CID 105024629.
- ^ Karousis N, Tagmatarchis N, Tasis D (September 2010). "Current progress on the chemical modification of carbon nanotubes". Chemical Reviews. 110 (9): 5366–5397. doi:10.1021/cr100018g. PMID 20545303.
- ^ Sadri R, Hosseini M, Kazi SN, Bagheri S, Zubir N, Solangi KH, et al. (October 2017). "A bio-based, facile approach for the preparation of covalently functionalized carbon nanotubes aqueous suspensions and their potential as heat transfer fluids". Journal of Colloid and Interface Science. 504: 115–123. Bibcode:2017JCIS..504..115S. doi:10.1016/j.jcis.2017.03.051. PMID 28531649.
- ^ Sahoo P, Shrestha RG, Shrestha LK, Hill JP, Takei T, Ariga K (November 2016). "Surface Oxidized Carbon Nanotubes Uniformly Coated with Nickel Ferrite Nanoparticles". Journal of Inorganic and Organometallic Polymers and Materials. 26 (6): 1301–1308. doi:10.1007/s10904-016-0365-z. S2CID 101287773.
- ^ Sahoo P, Tan JB, Zhang ZM, Singh SK, Lu TB (7 March 2018). "Engineering the Surface Structure of Binary/Ternary Ferrite Nanoparticles as High-Performance Electrocatalysts for the Oxygen Evolution Reaction". ChemCatChem. 10 (5): 1075–1083. doi:10.1002/cctc.201701790. S2CID 104164617.
- ^ US 10000382, Zaderko A, Vasyl UA, "Method for carbon materials surface modification by the fluorocarbons and derivatives", issued June 19, 2018 Archived 17 September 2018 at the Wayback Machine
- ^ "WO16072959 Method for Carbon Materials Surface Modification by the Fluorocarbons and Derivatives". patentscope.wipo.int. Retrieved 17 September 2018.
- ^ Sanei SH, Doles R, Ekaitis T (2019). "Effect of Nanocomposite Microstructure on Stochastic Elastic Properties: An Finite Element Analysis Study". ASCE-ASME Journal of Risk and Uncertainty in Engineering Systems, Part B: Mechanical Engineering. 5 (3): 030903. doi:10.1115/1.4043410. S2CID 140766023.
- ^ a b Stefaniak AB (2017). "Principal Metrics and Instrumentation for Characterization of Engineered Nanomaterials". In Mansfield E, Kaiser DL, Fujita D, Van de Voorde M (eds.). Metrology and Standardization of Nanotechnology. Wiley-VCH Verlag. pp. 151–174. doi:10.1002/9783527800308.ch8. ISBN 978-3-527-80030-8.
- ^ "ISO/TS 10868:2017 – Nanotechnologies – Characterization of single-wall carbon nanotubes using ultraviolet-visible-near infrared (UV-Vis-NIR) absorption spectroscopy". International Organization for Standardization. Archived from the original on 7 September 2017. Retrieved 6 September 2017.
- ^ "ISO/TS 10797:2012 – Nanotechnologies – Characterization of single-wall carbon nanotubes using transmission electron microscopy". International Organization for Standardization. Archived from the original on 7 September 2017. Retrieved 6 September 2017.
- ^ a b "ISO/TS 10798:2011 – Nanotechnologies – Characterization of single-wall carbon nanotubes using scanning electron microscopy and energy dispersive X-ray spectrometry analysis". International Organization for Standardization. Archived from the original on 7 September 2017. Retrieved 6 September 2017.
- ^ a b Fagan J (5 March 2009). "Carbon Nanotube Reference Materials". U.S. National Institute of Standards and Technology. Retrieved 6 September 2017.
- ^ "SRM 2483 – Single-Wall Carbon Nanotubes (Raw Soot)". U.S. National Institute of Standards and Technology. Archived from the original on 18 February 2013. Retrieved 6 September 2017.
- ^ "SWCNT-1: Single-Wall Carbon Nanotube Certified Reference Material – National Research Council Canada". Canadian National Research Council. 7 November 2014. Retrieved 6 September 2017.
- ^ "RM 8281 – Single-Wall Carbon Nanotubes (Dispersed, Three Length-Resolved Populations)". U.S. National Institute of Standards and Technology. Archived from the original on 1 April 2015. Retrieved 6 September 2017.
- ^ "ISO/TR 10929:2012 – Nanotechnologies – Characterization of multiwall carbon nanotube (MWCNT) samples". International Organization for Standardization. Archived from the original on 7 September 2017. Retrieved 6 September 2017.
- ^ "ISO/TS 11888:2017 –Nanotechnologies – Characterization of multiwall carbon nanotubes – Mesoscopic shape factors". International Organization for Standardization. Archived from the original on 7 September 2017. Retrieved 6 September 2017.
- ^ Fraser K, Kodali V, Yanamala N, Birch ME, Cena L, Casuccio G, et al. (December 2020). "Physicochemical characterization and genotoxicity of the broad class of carbon nanotubes and nanofibers used or produced in U.S. facilities". Particle and Fibre Toxicology. 17 (1): 62. Bibcode:2020PFTox..17...62F. doi:10.1186/s12989-020-00392-w. PMC 7720492. PMID 33287860.
- ^ Fraser K, Hubbs A, Yanamala N, Mercer RR, Stueckle TA, Jensen J, et al. (December 2021). "Histopathology of the broad class of carbon nanotubes and nanofibers used or produced in U.S. facilities in a murine model". Particle and Fibre Toxicology. 18 (1): 47. Bibcode:2021PFTox..18...47F. doi:10.1186/s12989-021-00440-z. PMC 8686255. PMID 34923995.
- ^ Barbarino M, Giordano A (March 2021). "Assessment of the Carcinogenicity of Carbon Nanotubes in the Respiratory System". Cancers. 13 (6): 1318. doi:10.3390/cancers13061318. PMC 7998467. PMID 33804168.
- ^ "CDC – NIOSH Numbered Publications: Current Intelligence Bulletins (CIB) – Sorted By Date, Descending Order Without Publication Numbers". www.cdc.gov. Retrieved 9 November 2022.
- ^ Howard J (April 2013). "Occupational exposure to carbon nanotubes and nanofibers". Current Intelligence Bulletin. No. 65. DHHS (NIOSH) Publication. doi:10.26616/NIOSHPUB2013145.
- ^ "ccupational exposure to carbon nanotubes and nanofibers". Current Intelligence Bulletin. No. 65. 14 July 2020. doi:10.26616/NIOSHPUB2013145.
- ^ Bishop L, Cena L, Orandle M, Yanamala N, Dahm MM, Birch ME, et al. (26 September 2017). "In Vivo Toxicity Assessment of Occupational Components of the Carbon Nanotube Life Cycle To Provide Context to Potential Health Effects". ACS Nano. 11 (9): 8849–8863. doi:10.1021/acsnano.7b03038. ISSN 1936-0851. PMID 28759202.
- ^ "ECHA CHEM". chem.echa.europa.eu. Retrieved 10 June 2024.
- ^ "REACH Registration Completed for Single-Wall Carbon Nanotubes". pcimag.com. PCI Mag. 16 October 2016. Archived from the original on 24 November 2016. Retrieved 24 November 2016.
- ^ He H, Pham-Huy LA, Dramou P, Xiao D, Zuo P, Pham-Huy C (2013). "Carbon Nanotubes: Applications in Pharmacy and Medicine". BioMed Research International. 2013: 1–12. doi:10.1155/2013/578290. PMC 3806157. PMID 24195076.
- ^ The Effectiveness Mechanisms of Carbon Nanotubes (CNTs) as Reinforcements for Magnesium-Based Composites for Biomedical Applications: A Review Nanomaterials 2024, 14, 756. https://doi.org/10.3390/nano14090756
 PMCID: PMC11085746
PMCID: PMC11085746  PMID 38727350
PMID 38727350
- ^ Carbon Nanotube (CNT) Encapsulated Magnesium-Based Nanocomposites to Improve Mechanical, Degradation and Antibacterial Performances for Biomedical Device Applications Coatings 2022, 12, 1589. https://doi.org/10.3390/coatings12101589

- ^ Zhao M, Su R, Ji L, Zhang Y, Wu H, Wen Z, et al. (22 January 2023). "The Biocompatibility and Antibacterial Properties of the CNTs-Doped Magnesium Based Composite Implants in a Long-Term Biodegradation Process". Journal of Nanomaterials. 2023 (1): 1–17. doi:10.1155/2023/5012576.
- ^ Francis AA, Abdel-Gawad SA, Shoeib MA (July 2021). "Toward CNT-reinforced chitosan-based ceramic composite coatings on biodegradable magnesium for surgical implants". Journal of Coatings Technology and Research. 18 (4): 971–988. doi:10.1007/s11998-021-00468-y.
- ^ Cognet L, Tsyboulski DA, Rocha JD, Doyle CD, Tour JM, Weisman RB (8 June 2007). "Stepwise Quenching of Exciton Fluorescence in Carbon Nanotubes by Single-Molecule Reactions". Science. 316 (5830): 1465–1468. arXiv:0707.3246. Bibcode:2007Sci...316.1465C. doi:10.1126/science.1141316. ISSN 0036-8075. PMID 17556581. S2CID 7476534.
- ^ a b Heller I, Janssens AM, Männik J, Minot ED, Lemay SG, Dekker C (1 February 2008). "Identifying the Mechanism of Biosensing with Carbon Nanotube Transistors". Nano Letters. 8 (2): 591–595. Bibcode:2008NanoL...8..591H. doi:10.1021/nl072996i. ISSN 1530-6984. PMID 18162002.
- ^ Zhang J, Boghossian AA, Barone PW, Rwei A, Kim JH, Lin D, et al. (26 January 2011). "Single Molecule Detection of Nitric Oxide Enabled by d(AT) 15 DNA Adsorbed to Near Infrared Fluorescent Single-Walled Carbon Nanotubes". Journal of the American Chemical Society. 133 (3): 567–581. doi:10.1021/ja1084942. ISSN 0002-7863. PMID 21142158.
- ^ Jin H, Heller DA, Kalbacova M, Kim JH, Zhang J, Boghossian AA, et al. (April 2010). "Detection of single-molecule H2O2 signalling from epidermal growth factor receptor using fluorescent single-walled carbon nanotubes". Nature Nanotechnology. 5 (4): 302–309. doi:10.1038/nnano.2010.24. ISSN 1748-3387. PMC 6438196. PMID 20208549.
- ^ a b c Kruss S, Salem DP, Vuković L, Lima B, Vander Ende E, Boyden ES, et al. (21 February 2017). "High-resolution imaging of cellular dopamine efflux using a fluorescent nanosensor array". Proceedings of the National Academy of Sciences. 114 (8): 1789–1794. Bibcode:2017PNAS..114.1789K. doi:10.1073/pnas.1613541114. ISSN 0027-8424. PMC 5338365. PMID 28179565.
- ^ a b Wu H, Nißler R, Morris V, Herrmann N, Hu P, Jeon SJ, et al. (8 April 2020). "Monitoring Plant Health with Near-Infrared Fluorescent H 2 O 2 Nanosensors". Nano Letters. 20 (4): 2432–2442. Bibcode:2020NanoL..20.2432W. doi:10.1021/acs.nanolett.9b05159. ISSN 1530-6984. PMID 32097014. S2CID 211524215.
- ^ a b Lew TT, Koman VB, Silmore KS, Seo JS, Gordiichuk P, Kwak SY, et al. (15 April 2020). "Real-time detection of wound-induced H2O2 signalling waves in plants with optical nanosensors". Nature Plants. 6 (4): 404–415. doi:10.1038/s41477-020-0632-4. ISSN 2055-0278. PMID 32296141. S2CID 215774820.
- ^ Jin H, Heller DA, Kim JH, Strano MS (10 December 2008). "Stochastic Analysis of Stepwise Fluorescence Quenching Reactions on Single-Walled Carbon Nanotubes: Single Molecule Sensors". Nano Letters. 8 (12): 4299–4304. Bibcode:2008NanoL...8.4299J. doi:10.1021/nl802010z. ISSN 1530-6984. PMID 19367966.
- ^ Giraldo JP, Landry MP, Kwak SY, Jain RM, Wong MH, Iverson NM, et al. (August 2015). "A Ratiometric Sensor Using Single Chirality Near-Infrared Fluorescent Carbon Nanotubes: Application to In Vivo Monitoring". Small. 11 (32): 3973–3984. doi:10.1002/smll.201403276. hdl:1721.1/102316. ISSN 1613-6810. PMID 25981520. S2CID 44726670.
- ^ Dinarvand M, Neubert E, Meyer D, Selvaggio G, Mann FA, Erpenbeck L, et al. (11 September 2019). "Near-Infrared Imaging of Serotonin Release from Cells with Fluorescent Nanosensors". Nano Letters. 19 (9): 6604–6611. Bibcode:2019NanoL..19.6604D. doi:10.1021/acs.nanolett.9b02865. ISSN 1530-6984. PMID 31418577. S2CID 201019834.
- ^ Jeong S, Yang D, Beyene AG, Del Bonis-O'Donnell JT, Gest AM, Navarro N, et al. (6 December 2019). "High-throughput evolution of near-infrared serotonin nanosensors". Science Advances. 5 (12): eaay3771. Bibcode:2019SciA....5.3771J. doi:10.1126/sciadv.aay3771. ISSN 2375-2548. PMC 6920020. PMID 31897432.
- ^ Manoharan G, Bösel P, Thien J, Holtmannspötter M, Meingast L, Schmidt M, et al. (25 February 2023). "Click-Functionalization of Silanized Carbon Nanotubes: From Inorganic Heterostructures to Biosensing Nanohybrids". Molecules. 28 (5): 2161. doi:10.3390/molecules28052161. ISSN 1420-3049. PMC 10004328. PMID 36903408.
- ^ Del Bonis-O'Donnell JT, Pinals RL, Jeong S, Thakrar A, Wolfinger RD, Landry MP (8 January 2019). "Chemometric Approaches for Developing Infrared Nanosensors To Image Anthracyclines". Biochemistry. 58 (1): 54–64. doi:10.1021/acs.biochem.8b00926. ISSN 0006-2960. PMC 6411385. PMID 30480442.
- ^ Wong MH, Giraldo JP, Kwak SY, Koman VB, Sinclair R, Lew TT, et al. (February 2017). "Nitroaromatic detection and infrared communication from wild-type plants using plant nanobionics". Nature Materials. 16 (2): 264–272. Bibcode:2017NatMa..16..264W. doi:10.1038/nmat4771. ISSN 1476-1122. PMID 27798623.
- ^ a b Xu X, Clément P, Eklöf-Österberg J, Kelley-Loughnane N, Moth-Poulsen K, Chávez JL, et al. (11 July 2018). "Reconfigurable Carbon Nanotube Multiplexed Sensing Devices". Nano Letters. 18 (7): 4130–4135. Bibcode:2018NanoL..18.4130X. doi:10.1021/acs.nanolett.8b00856. ISSN 1530-6984. PMID 29923734. S2CID 49310769.
- ^ Jena PV, Roxbury D, Galassi TV, Akkari L, Horoszko CP, Iaea DB, et al. (28 November 2017). "A Carbon Nanotube Optical Reporter Maps Endolysosomal Lipid Flux". ACS Nano. 11 (11): 10689–10703. doi:10.1021/acsnano.7b04743. ISSN 1936-0851. PMC 5707631. PMID 28898055.
- ^ Galassi TV, Jena PV, Shah J, Ao G, Molitor E, Bram Y, et al. (3 October 2018). "An optical nanoreporter of endolysosomal lipid accumulation reveals enduring effects of diet on hepatic macrophages in vivo". Science Translational Medicine. 10 (461). doi:10.1126/scitranslmed.aar2680. ISSN 1946-6234. PMC 6543545. PMID 30282694.
- ^ Bisker G, Dong J, Park HD, Iverson NM, Ahn J, Nelson JT, et al. (8 January 2016). "Protein-targeted corona phase molecular recognition". Nature Communications. 7 (1): 10241. Bibcode:2016NatCo...710241B. doi:10.1038/ncomms10241. ISSN 2041-1723. PMC 4729864. PMID 26742890.
- ^ Kim J, Campbell AS, de Ávila BE, Wang J (April 2019). "Wearable biosensors for healthcare monitoring". Nature Biotechnology. 37 (4): 389–406. doi:10.1038/s41587-019-0045-y. ISSN 1087-0156. PMC 8183422. PMID 30804534.
- ^ Barone PW, Strano MS (11 December 2006). "Reversible Control of Carbon Nanotube Aggregation for a Glucose Affinity Sensor". Angewandte Chemie International Edition. 45 (48): 8138–8141. doi:10.1002/anie.200603138. ISSN 1433-7851. PMID 17099921.
- ^ a b Zubkovs V, Wang H, Schuergers N, Weninger A, Glieder A, Cattaneo S, et al. (2022). "Bioengineering a glucose oxidase nanosensor for near-infrared continuous glucose monitoring". Nanoscale Advances. 4 (11): 2420–2427. Bibcode:2022NanoA...4.2420Z. doi:10.1039/D2NA00092J. ISSN 2516-0230. PMC 9154020. PMID 35746900.
- ^ Harvey JD, Jena PV, Baker HA, Zerze GH, Williams RM, Galassi TV, et al. (13 March 2017). "A carbon nanotube reporter of microRNA hybridization events in vivo". Nature Biomedical Engineering. 1 (4). doi:10.1038/s41551-017-0041. ISSN 2157-846X. PMC 5568023. PMID 28845337.
- ^ Harvey JD, Baker HA, Ortiz MV, Kentsis A, Heller DA (24 May 2019). "HIV Detection via a Carbon Nanotube RNA Sensor". ACS Sensors. 4 (5): 1236–1244. doi:10.1021/acssensors.9b00025. ISSN 2379-3694. PMC 7556989. PMID 31056899.
- ^ Kallmyer NE, Abdennadher MS, Agarwal S, Baldwin-Kordick R, Khor RL, Kooistra AS, et al. (23 March 2021). "Inexpensive Near-Infrared Fluorimeters: Enabling Translation of nIR-Based Assays to the Field". Analytical Chemistry. 93 (11): 4800–4808. doi:10.1021/acs.analchem.0c03732. ISSN 0003-2700. PMID 33703890. S2CID 232188200.
- ^ Shumeiko V, Paltiel Y, Bisker G, Hayouka Z, Shoseyov O (14 September 2020). "A Paper-Based Near-Infrared Optical Biosensor for Quantitative Detection of Protease Activity Using Peptide-Encapsulated SWCNTs". Sensors. 20 (18): 5247. Bibcode:2020Senso..20.5247S. doi:10.3390/s20185247. ISSN 1424-8220. PMC 7570893. PMID 32937986.
- ^ a b c d e Nißler R, Bader O, Dohmen M, Walter SG, Noll C, Selvaggio G, et al. (25 November 2020). "Remote near infrared identification of pathogens with multiplexed nanosensors". Nature Communications. 11 (1): 5995. Bibcode:2020NatCo..11.5995N. doi:10.1038/s41467-020-19718-5. ISSN 2041-1723. PMC 7689463. PMID 33239609.
- ^ Clément P, Ackermann J, Sahin-Solmaz N, Herbertz S, Boero G, Kruss S, et al. (November 2022). "Comparison of electrical and optical transduction modes of DNA-wrapped SWCNT nanosensors for the reversible detection of neurotransmitters". Biosensors and Bioelectronics. 216: 114642. doi:10.1016/j.bios.2022.114642. PMID 36055131.
- ^ O'Connell MJ, Bachilo SM, Huffman CB, Moore VC, Strano MS, Haroz EH, et al. (26 July 2002). "Band Gap Fluorescence from Individual Single-Walled Carbon Nanotubes". Science. 297 (5581): 593–596. Bibcode:2002Sci...297..593O. doi:10.1126/science.1072631. ISSN 0036-8075. PMID 12142535. S2CID 22623119.
- ^ Xu B, Wu X, Kim M, Wang P, Wang Y (28 January 2021). "Electroluminescence from 4-nitroaryl organic color centers in semiconducting single-wall carbon nanotubes". Journal of Applied Physics. 129 (4): 044305. Bibcode:2021JAP...129d4305X. doi:10.1063/5.0039047. ISSN 0021-8979.
- ^ Li MK, Riaz A, Wederhake M, Fink K, Saha A, Dehm S, et al. (23 August 2022). "Electroluminescence from Single-Walled Carbon Nanotubes with Quantum Defects". ACS Nano. 16 (8): 11742–11754. doi:10.1021/acsnano.2c03083. ISSN 1936-0851. OSTI 1879407. PMID 35732039. S2CID 249956650.
- ^ Hong G, Antaris AL, Dai H (10 January 2017). "Near-infrared fluorophores for biomedical imaging". Nature Biomedical Engineering. 1 (1). doi:10.1038/s41551-016-0010. ISSN 2157-846X. S2CID 78795936.
- ^ Hong G, Diao S, Antaris AL, Dai H (14 October 2015). "Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy". Chemical Reviews. 115 (19): 10816–10906. doi:10.1021/acs.chemrev.5b00008. ISSN 0009-2665. PMID 25997028.
- ^ Dong J, Salem DP, Sun JH, Strano MS (24 April 2018). "Analysis of Multiplexed Nanosensor Arrays Based on Near-Infrared Fluorescent Single-Walled Carbon Nanotubes". ACS Nano. 12 (4): 3769–3779. doi:10.1021/acsnano.8b00980. ISSN 1936-0851. PMID 29614219.
- ^ Salem DP, Gong X, Liu AT, Akombi K, Strano MS (7 January 2020). "Immobilization and Function of nIR-Fluorescent Carbon Nanotube Sensors on Paper Substrates for Fluidic Manipulation". Analytical Chemistry. 92 (1): 916–923. doi:10.1021/acs.analchem.9b03756. ISSN 0003-2700. PMID 31829619. S2CID 209340238.
- ^ Roxbury D, Jena PV, Williams RM, Enyedi B, Niethammer P, Marcet S, et al. (21 September 2015). "Hyperspectral Microscopy of Near-Infrared Fluorescence Enables 17-Chirality Carbon Nanotube Imaging". Scientific Reports. 5 (1): 14167. Bibcode:2015NatSR...514167R. doi:10.1038/srep14167. ISSN 2045-2322. PMC 4585673. PMID 26387482.
- ^ Wei X, Tanaka T, Akizuki N, Miyauchi Y, Matsuda K, Ohfuchi M, et al. (19 May 2016). "Single-Chirality Separation and Optical Properties of (5,4) Single-Wall Carbon Nanotubes". The Journal of Physical Chemistry C. 120 (19): 10705–10710. doi:10.1021/acs.jpcc.6b03257. ISSN 1932-7447.
- ^ Selvaggio G, Chizhik A, Nißler R, Kuhlemann l, Meyer D, Vuong L, et al. (20 March 2020). "Exfoliated near infrared fluorescent silicate nanosheets for (bio)photonics". Nature Communications. 11 (1): 1495. Bibcode:2020NatCo..11.1495S. doi:10.1038/s41467-020-15299-5. ISSN 2041-1723. PMC 7083911. PMID 32198383.
- ^ Sistemich L, Galonska P, Stegemann J, Ackermann J, Kruss S (12 June 2023). "Near-Infrared Fluorescence Lifetime Imaging of Biomolecules with Carbon Nanotubes**". Angewandte Chemie International Edition. 62 (24): e202300682. doi:10.1002/anie.202300682. ISSN 1433-7851. PMID 36891826.
- ^ Kruss S, Hilmer AJ, Zhang J, Reuel NF, Mu B, Strano MS (December 2013). "Carbon nanotubes as optical biomedical sensors". Advanced Drug Delivery Reviews. 65 (15): 1933–1950. doi:10.1016/j.addr.2013.07.015. PMID 23906934.
- ^ Boghossian AA, Zhang J, Barone PW, Reuel NF, Kim JH, Heller DA, et al. (18 July 2011). "Near-Infrared Fluorescent Sensors based on Single-Walled Carbon Nanotubes for Life Sciences Applications". ChemSusChem. 4 (7): 848–863. Bibcode:2011ChSCh...4..848B. doi:10.1002/cssc.201100070. ISSN 1864-5631. PMID 21751417.
- ^ Nißler R, Müller AT, Dohrman F, Kurth L, Li H, Cosio EG, et al. (10 January 2022). "Detection and Imaging of the Plant Pathogen Response by Near-Infrared Fluorescent Polyphenol Sensors". Angewandte Chemie International Edition. 61 (2): e202108373. doi:10.1002/anie.202108373. ISSN 1433-7851. PMC 9298901. PMID 34608727.
- ^ Elizarova S, Chouaib AA, Shaib A, Hill B, Mann F, Brose N, et al. (31 May 2022). "A fluorescent nanosensor paint detects dopamine release at axonal varicosities with high spatiotemporal resolution". Proceedings of the National Academy of Sciences. 119 (22): e2202842119. Bibcode:2022PNAS..11902842E. doi:10.1073/pnas.2202842119. ISSN 0027-8424. PMC 9295782. PMID 35613050.
- ^ Gerstman E, Hendler-Neumark A, Wulf V, Bisker G (10 May 2023). "Monitoring the Formation of Fibrin Clots as Part of the Coagulation Cascade Using Fluorescent Single-Walled Carbon Nanotubes". ACS Applied Materials & Interfaces. 15 (18): 21866–21876. doi:10.1021/acsami.3c00828. ISSN 1944-8244. PMC 10176323. PMID 37128896.
- ^ Ehrlich R, Hendler-Neumark A, Wulf V, Amir D, Bisker G (July 2021). "Optical Nanosensors for Real-Time Feedback on Insulin Secretion by β-Cells". Small. 17 (30): e2101660. doi:10.1002/smll.202101660. ISSN 1613-6810. PMID 34197026.
- ^ Kim M, Chen C, Wang P, Mulvey JJ, Yang Y, Wun C, et al. (17 March 2022). "Detection of ovarian cancer via the spectral fingerprinting of quantum-defect-modified carbon nanotubes in serum by machine learning". Nature Biomedical Engineering. 6 (3): 267–275. doi:10.1038/s41551-022-00860-y. ISSN 2157-846X. PMC 9108893. PMID 35301449.
- ^ Metternich JT, Wartmann JA, Sistemich L, Nißler R, Herbertz S, Kruss S (12 July 2023). "Near-Infrared Fluorescent Biosensors Based on Covalent DNA Anchors". Journal of the American Chemical Society. 145 (27): 14776–14783. doi:10.1021/jacs.3c03336. ISSN 0002-7863. PMID 37367958. S2CID 259261621.
- ^ Ackermann J, Reger E, Jung S, Mohr J, Herbertz S, Seidl K, et al. (February 2024). "Smart Slides for Optical Monitoring of Cellular Processes". Advanced Functional Materials. 34 (6). doi:10.1002/adfm.202309064. ISSN 1616-301X.
- ^ "Smart Nanotubes – Gas sensor development". Smart Nanotubes. Retrieved 9 February 2024.
- ^ "Welcome". www.zymosense.com. Retrieved 9 February 2024.
- ^ Pagni J (5 March 2010). "Amroy aims to become nano-leader". European Plastics News. Archived from the original on 10 July 2011.
- ^ "Carbon nanotube tape stays sticky in extreme temperatures". Nanowerk Newsletter. American Chemical Society. 10 July 2019.
- ^ "Nanotube Tips". nanoScience instruments. Archived from the original on 27 October 2011.
- ^ "Nanotube solution for medical devices approved to enter EU market". Med-Tech Innovation. 21 September 2022. Retrieved 6 September 2024.
- ^ Kim MJ, Kim H, Kim J, Lee YJ, Lee W, Hwang JY, et al. (28 June 2024). "Molecular-level hybridization of single-walled carbon nanotubes and a copper complex with counterbalanced electrostatic interactions". Communications Materials. 5 (1): 111. Bibcode:2024CoMat...5..111K. doi:10.1038/s43246-024-00548-7. ISSN 2662-4443.
- ^ Paleo AJ, Martinez-Rubi Y, Krause B, Pötschke P, Jakubinek MB, Ashrafi B, et al. (13 October 2023). "Carbon Nanotube–Polyurethane Composite Sheets for Flexible Thermoelectric Materials". ACS Applied Nano Materials. 6 (19): 17986–17995. doi:10.1021/acsanm.3c03247. ISSN 2574-0970. PMC 10580240. PMID 37854856.
- ^ Tiza MT (6 December 2022). "Applications of nanomaterials in highway pavements". NanoEra (2(2), 23–29) – via Atatürk University Press.
- ^ Demski S, Misiak M, Majchrowicz K, Komorowska G, Lipkowski A, Stankiewicz K, et al. (21 May 2024). "Mechanical recycling of CFRPs based on thermoplastic acrylic resin with the addition of carbon nanotubes". Scientific Reports. 14 (1): 11550. Bibcode:2024NatSR..1411550D. doi:10.1038/s41598-024-62594-y. ISSN 2045-2322. PMC 11109235. PMID 38773242.
- ^ Nechausov S, Miriyev A (15 September 2024). "3D-Printable high-mixed-conductivity ionogel composites for soft multifunctional devices". Chemical Engineering Journal. 496: 153759. Bibcode:2024ChEnJ.49653759N. doi:10.1016/j.cej.2024.153759. ISSN 1385-8947.
- ^ Noyce SG, Doherty JL, Cheng Z, Han H, Bowen S, Franklin AD (March 2019). "Electronic Stability of Carbon Nanotube Transistors Under Long-Term Bias Stress". Nano Letters. 19 (3): 1460–1466. Bibcode:2019NanoL..19.1460N. doi:10.1021/acs.nanolett.8b03986. PMID 30720283. S2CID 73450707.
- ^ "Publications on carbon nanotube applications including scaffold microfabrication". nano.byu.edu. 27 May 2014.
- ^ Belkin A, Hubler A, Bezryadin A (February 2015). "Self-assembled wiggling nano-structures and the principle of maximum entropy production". Scientific Reports. 5: 8323. Bibcode:2015NatSR...5.8323B. doi:10.1038/srep08323. PMC 4321171. PMID 25662746.
- ^ Tan CW, Tan KH, Ong YT, Mohamed AR, Zein SH, Tan SH (September 2012). "Energy and environmental applications of carbon nanotubes". Environmental Chemistry Letters. 10 (3): 265–273. Bibcode:2012EnvCL..10..265T. doi:10.1007/s10311-012-0356-4. S2CID 95369378.
- ^ Tucker A. "Jack Andraka, the Teen Prodigy of Pancreatic Cancer". Smithsonian Magazine. Retrieved 2 March 2021.
- ^ [1]US 9329021, DeLuca MJ, Felker CJ, Heider D, "System and methods for use in monitoring a structure", published May 3, 2016
- ^ "Pirahna USV built using nano-enhanced carbon prepreg". ReinforcedPlastics.com. 19 February 2009. Archived from the original on 3 March 2012.
- ^ LaPedus M (22 March 2021). "EUV Pellicles Finally Ready". Semiconductor Engineering. Retrieved 13 November 2022.
- ^ Zanello LP, Zhao B, Hu H, Haddon RC (March 2006). "Bone cell proliferation on carbon nanotubes". Nano Letters. 6 (3): 562–567. Bibcode:2006NanoL...6..562Z. doi:10.1021/nl051861e. PMID 16522063.
- ^ "Legendary Swords' Sharpness, Strength From Nanotubes, Study Says". news.nationalgeographic.com. Archived from the original on 18 November 2006.
- ^ Gullapalli S, Wong MS (2011). "Nanotechnology: A Guide to Nano-Objects" (PDF). Chemical Engineering Progress. 107 (5): 28–32. Archived from the original (PDF) on 13 August 2012. Retrieved 24 November 2011.
- ^ Simonite T. "IBM Expects Nanotube Transistor Computer Chips Ready Soon After 2020". MIT Technology Review.
- ^ Billington J (4 August 2022). "Faster charging and more powerful EV batteries boosted by graphene nanotube production". Electric & Hybrid Vehicle Technology International. Retrieved 7 August 2024.
- ^ Moore S (31 August 2020). "Graphene Nanotubes Make Polyamide Paintable". Plastics Today. Retrieved 7 August 2024.
- ^ "Clear Skies Coatings Presented a Waterborne Conductive Primer & Adhesion Promoter with Graphene Nanotubes – IPCM". www.ipcm.it. Retrieved 7 August 2024.
- ^ "Overcoming ESD-Control Flooring Challenges: A Comprehensive Guide to ANSI/ESD S20.20-2021 | FLOOR Trends & Installation". www.floortrendsmag.com. Retrieved 7 August 2024.
- ^ Soto Beobide A, Bieri R, Szakács Z, Sparwasser K, Kaitsa IG, Georgiopoulos I, et al. (January 2024). "Raman Spectroscopy Unfolds the Fate and Transformation of SWCNTs after Abrasive Wear of Epoxy Floor Coatings". Nanomaterials. 14 (1): 120. doi:10.3390/nano14010120. ISSN 2079-4991. PMC 10780583. PMID 38202575.
- ^ "Graphene goes industrial without a bang". www.compositesworld.com. 26 July 2024. Retrieved 7 August 2024.
- ^ "OCSiAl, Daikin improve fluoropolymer resistance to extreme conditions | European Rubber Journal". www.european-rubber-journal.com. Retrieved 7 August 2024.
- ^ Liu G, Wang H, Ren T, Chen Y, Liu S (January 2024). "Systematic Investigation of the Degradation Properties of Nitrile-Butadiene Rubber/Polyamide Elastomer/Single-Walled Carbon Nanotube Composites in Thermo-Oxidative and Hot Oil Environments". Polymers. 16 (2): 226. doi:10.3390/polym16020226. ISSN 2073-4360. PMC 10820770. PMID 38257025.
- ^ "BÜFA releases line of novel conductive gelcoats". www.compositesworld.com. 26 July 2024. Retrieved 7 August 2024.
- ^ "Polymer fibers with graphene nanotubes make it possible to heat hard-to-reach, complex-shaped items – Modern Plastics India". 21 February 2022. Retrieved 7 August 2024.
- ^ Thomas DJ (June 2018). "Ultrafine graphitised MWCNT nanostructured yarn for the manufacture of electrically conductive fabric". The International Journal of Advanced Manufacturing Technology. 96 (9–12): 3805–3808. doi:10.1007/s00170-017-1320-z. S2CID 115751858.
- ^ Sanderson K (2006). "Sharpest cut from nanotube sword". Nature News. doi:10.1038/news061113-11. S2CID 136774602.
- ^ Reibold M, Paufler P, Levin AA, Kochmann W, Pätzke N, Meyer DC (November 2006). "Materials: carbon nanotubes in an ancient Damascus sabre". Nature. 444 (7117): 286. Bibcode:2006Natur.444..286R. doi:10.1038/444286a. PMID 17108950. S2CID 4431079.
- ^ Valenti G, Boni A, Melchionna M, Cargnello M, Nasi L, Bertoni G, et al. (December 2016). "Co-axial heterostructures integrating palladium/titanium dioxide with carbon nanotubes for efficient electrocatalytic hydrogen evolution". Nature Communications. 7: 13549. Bibcode:2016NatCo...713549V. doi:10.1038/ncomms13549. PMC 5159813. PMID 27941752.
- ^ a b Lienig J, Thiele M (2018). "Mitigating Electromigration in Physical Design". Fundamentals of Electromigration-Aware Integrated Circuit Design. Springer. pp. 138–140. doi:10.1007/978-3-319-73558-0. ISBN 978-3-319-73557-3.
- ^ Dekker C (May 1999). "Carbon Nanotubes as Molecular Quantum Wires". Physics Today. 52 (5): 22–28. Bibcode:1999PhT....52e..22D. doi:10.1063/1.882658.
- ^ Martel R, Derycke V, Lavoie C, Appenzeller J, Chan KK, Tersoff J, et al. (December 2001). "Ambipolar electrical transport in semiconducting single-wall carbon nanotubes". Physical Review Letters. 87 (25): 256805. Bibcode:2001PhRvL..87y6805M. doi:10.1103/PhysRevLett.87.256805. PMID 11736597.
- ^ Susantyoko RA, Karam Z, Alkhoori S, Mustafa I, Wu CH, Almheiri S (2017). "A surface-engineered tape-casting fabrication technique toward the commercialisation of freestanding carbon nanotube sheets". Journal of Materials Chemistry A. 5 (36): 19255–19266. doi:10.1039/c7ta04999d.
- ^ Karam Z, Susantyoko RA, Alhammadi A, Mustafa I, Wu CH, Almheiri S (June 2018). "Development of Surface-Engineered Tape-Casting Method for Fabricating Freestanding Carbon Nanotube Sheets Containing Fe2O3 Nanoparticles for Flexible Batteries". Advanced Engineering Materials. 20 (6): 1701019. doi:10.1002/adem.201701019. S2CID 139283096.
- ^ Behabtu N, Young CC, Tsentalovich DE, Kleinerman O, Wang X, Ma AW, et al. (January 2013). "Strong, light, multifunctional fibers of carbon nanotubes with ultrahigh conductivity". Science. 339 (6116): 182–186. Bibcode:2013Sci...339..182B. doi:10.1126/science.1228061. hdl:1911/70792. PMID 23307737. S2CID 10843825.
- ^ Piraux L, Araujo FA, Bui TN, Otto MJ, Issi JP (26 August 2015). "Two-dimensional quantum transport in highly conductive carbon nanotube fibers". Physical Review B. 92 (8): 085428. Bibcode:2015PhRvB..92h5428P. doi:10.1103/PhysRevB.92.085428.
- ^ Liu F, Wagterveld RM, Gebben B, Otto MJ, Biesheuvel PM, Hamelers HV (November 2014). "Carbon nanotube yarns as strong flexible conductive capacitive electrodes". Colloid and Interface Science Communications. 3: 9–12. doi:10.1016/j.colcom.2015.02.001.
- ^ Pyrhönen J, Montonen J, Lindh P, Vauterin J, Otto M (28 February 2015). "Replacing Copper with New Carbon Nanomaterials in Electrical Machine Windings". International Review of Electrical Engineering. 10 (1): 12. CiteSeerX 10.1.1.1005.8294. doi:10.15866/iree.v10i1.5253.
- ^ Carbon Nanotube Yarn Rotates Electric Motors at LUT. YouTube
External links
[edit]- Nanocarbon: From Graphene to Buckyballs. Interactive 3D models of cyclohexane, benzene, graphene, graphite, chiral & non-chiral nanotubes, and C60 Buckyballs – WeCanFigureThisOut.org.
- C60 and Carbon Nanotubes a short video explaining how nanotubes can be made from modified graphite sheets and the three different types of nanotubes that are formed
- Learning module for Bandstructure of Carbon Nanotubes and Nanoribbons
- Selection of free-download articles on carbon nanotubes
- WOLFRAM Demonstrations Project: Electronic Band Structure of a Single-Walled Carbon Nanotube by the Zone-Folding Method
- WOLFRAM Demonstrations Project: Electronic Structure of a Single-Walled Carbon Nanotube in Tight-Binding Wannier Representation