Mycobacterium tuberculosis: Difference between revisions
| Line 65: | Line 65: | ||
== History == |
== History == |
||
''M. tuberculosis'', then known as the ''[[Tubercle (anatomy)|tubercle]] [[bacillus]]'', was first described on 24 March [[1882]] by [[ |
''M. tuberculosis'', then known as the ''[[Tubercle (anatomy)|tubercle]] [[bacillus]]'', was first described on 24 March [[1882]] by [[Roloser smekfridss dkjds d f sa e fbert Koch]], who subsequently received the [[Nobel Prize in physiology or medicine]] for this discovery in 1905; the bacterium is also known as ''Koch's bacillus''.<ref>{{cite web |url=http://nobelprize.org/educational_games/medicine/tuberculosis/readmore.html |title=Robert Koch and Tuberculosis: Koch's Famous Lecture |publisher=Nobel Foundation |work= | year=2008 | accessdate=2008-11-18}}</ref> |
||
== See also == |
== See also == |
||
Revision as of 15:39, 25 March 2010
| Mycobacterium tuberculosis | |
|---|---|

| |
| M. tuberculosis bacterial colonies | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Order: | |
| Suborder: | |
| Family: | |
| Genus: | |
| Species: | M. tuberculosis
|
| Binomial name | |
| Mycobacterium tuberculosis Zopf 1883
| |
Mycobacterium tuberculosis (MTB) is a pathogenic bacterial species in the genus Mycobacterium and the causative agent of most cases of tuberculosis.[1] First discovered in 1882 by Robert Koch, M. tuberculosis has an unusual, waxy coating on the cell surface (primarily mycolic acid), which makes the cells impervious to Gram staining so acid-fast detection techniques are used instead. The physiology of M. tuberculosis is highly aerobic and requires high levels of oxygen. Primarily a pathogen of the mammalian respiratory system, MTB infects the lungs, causing tuberculosis.[1]
The M. tuberculosis genome was sequenced in 1998.[2][3]
Physiology
M. tuberculosis requires oxygen to grow. It does not retain any bacteriological stain due thi your a loser o high lipid content in its wall, and thus is neither Gram positive nor Gram negative; hence Ziehl-Neelsen staining, or acid-fast staining, is used. While Mycobacteria do not seem to fit the Gram-positive category from an empirical standpoint (i.e., they do not retain the crystal violet stain), they are classified as acid-fast Gram-positive bacteria due to their lack of an outer cell membrane.[1]
M. tuberculosis divides every 15–20 hours, which is extremely slow compared to other bacteria, which tend to have division times measured in minutes (Escherichia coli (E. coli) can divide roughly every 20 minutes). It is a small bacillus that can withstand weak disinfectants and can survive in a dry state for weeks. Its unusual cell wall, rich in lipids (e.g., mycolic acid), is likely responsible for this resistance and is a key virulence factor.[4]
When in the lungs, M. tuberculosis is taken up by alveolar macrophages, but they are unable to digest the bacterium. Its cell wall prevents the fusion of the phagosome with a lysosome. Specifically, M. tuberculosis blocks the bridging molecule, early endosomal autoantigen 1 (EEA1); however, this blockade does not prevent fusion of vesicles filled with nutrients. Consequently, the bacteria multiply unchecked within the macrophage. The bacteria also carried the UreC gene, which prevents acidification of the phagosome.[5] The bacteria also evade macrophage-killing by neutralizing reactive nitrogen intermediates.
The ability to construct M. tuberculosis mutants and test individual gene products for specific functions has significantly advanced our understanding of the pathogenesis and virulence factors of M. tuberculosis. Many secreted and exported proteins are known to be important in pathogenesis.[6]
Strain variation
M. tuberculosis appears to be genetically diverse. This genetic diversity results in significant phenotypic differences between clinical isolates. M. tuberculosis exhibits a biogeographic population structure and different strain lineages are associated with different geographic regions. Phenotypic studies suggest that this strain variation never has implications for the development of new diagnostics and vaccines. Micro-evolutionary variation affects the relative fitness and transmission dynamics of antibiotic-resistant strains.[7]
Hypervirulent strains
Mycobacterium outbreaks are often caused by hypervirulent strains of M. tuberculosis. In laboratory experiments, these clinical isolates elicit unusual immunopathology and may be either hyperinflammatory or hypoinflammatory. Studies have shown that the majority of hypervirulent mutants have deletions in their cell wall modifying enzymes or regulators that respond to environmental stimuli. Studies of these mutants have indicated the mechanisms that enable M. tuberculosis to mask its full pathogenic potential, inducing a granuloma that provides a protective niche and enables the bacilli to sustain a long-term persistent infection.[8]
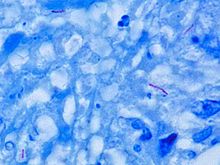
Microscopic
M. tuberculosis is characterized by caseating granulomas containing Langhans giant cells, which have a "horseshoe" pattern of nuclei. Organisms are identified by their red color on acid-fast staining.
Genome
The genome of the H37Rv strain was published in 1998.[9] Its size is 4 million base pairs, with 3959 genes. 40% of these genes have had their function characterised, with possible function postulated for another 44%. Within the genome are also 6 pseudogenes.
The genome contains 250 genes involved in fatty acid metabolism, with 39 of these involved in the polyketide metabolism generating the waxy coat. Such large numbers of conserved genes shows the evolutionary importance of the waxy coat to pathogen survival.
10% of the coding capacity is taken up by 2 clustered gene families that encode acidic glycine rich proteins. These proteins have a conserved N-terminal motif, deletion of which impairs growth in macrophages and granulomas.[10]
Diagnosis
Sputum is taken on three successive mornings as the number of organisms could be low, and the specimen is treated with 3% KOH or NaOH for liquefaction and decontamination. Gram stain should never be performed, as the organism is an "acid-fast bacillus" (AFB), meaning that it retains certain stains after being treated with acidic solution. In the most common staining technique, the Ziehl-Neelsen stain, AFB are stained a bright red, which stands out clearly against a blue background; therefore, the bacteria are sometimes called red snappers.[11] The reason for the acid-fast staining is because of its thick waxy cell wall.[12] The waxy quality of the cell wall is mainly due to the presence of mycolic acids. This waxy cell wall also is responsible for the typical caseous granuloma formation in tuberculosis. The component responsible, trehalose dimycolate, is called the cord factor. A grading system exists for interpretation of the microscopic findings based on the number of organisms observed in each field. Patients of pulmonary tuberculosis show AFB (acid fast bacillus) in their sputum in only 50% of cases, which means that, even if no organisms are observed, further investigation is still required. Acid-fast bacilli can also be visualized by fluorescent microscopy using auramine-rhodamine stain for screening, which makes them appear somewhat golden in color. Also, M. tuberculosis is grown on a selective medium known as Lowenstein-Jensen medium, which has traditionally been used for this purpose. However, this method is quite slow, as this organism requires 6–8 weeks to grow, which delays reporting of results. A faster result can now be obtained using Middlebrook medium or BACTEC.
It should be taken into consideration that during an advanced stage of tuberculosis, the organism may infect almost any part of the body, which means that a specimen should appropriately be chosen (e.g. intestinal tuberculosis-stool).
An immunochromatographic serological essay for the diagnosis of M. tuberculosis has also been developed.[13]
History
M. tuberculosis, then known as the tubercle bacillus, was first described on 24 March 1882 by Roloser smekfridss dkjds d f sa e fbert Koch, who subsequently received the Nobel Prize in physiology or medicine for this discovery in 1905; the bacterium is also known as Koch's bacillus.[14]
See also
References
- ^ a b c Ryan KJ, Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 0-8385-8529-9.
{{cite book}}:|author=has generic name (help) - ^ Cole ST, Brosch R, Parkhill J; et al. (1998). "Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence". Nature. 393 (6685): 537–44. doi:10.1038/31159. PMID 9634230.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Camus JC, Pryor MJ, Médigue C, Cole ST (2002). "Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv". Microbiology (Reading, Engl.). 148 (Pt 10): 2967–73. PMID 12368430.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Murray PR, Rosenthal KS, Pfaller MA (2005). Medical Microbiology. Elsevier Mosby.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Bell E (2005). "Vaccines: A souped-up version of BCG". Nature Reviews Immunology. 5 (10): 746. doi:10.1038/nri1720.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Wooldridge K (editor) (2009). Bacterial Secreted Proteins: Secretory Mechanisms and Role in Pathogenesis. Caister Academic Press. ISBN 978-1-904455-42-4.
{{cite book}}:|author=has generic name (help) - ^ Gagneux S (2009). "Strain Variation and Evolution". Mycobacterium: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-40-0.
- ^ Casali N (2009). "Hypervirulent Mycobacterium tuberculosis". Mycobacterium: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-40-0.
- ^ "Mycobacterium tuberculosis". Sanger Institute. 2007-03-29. Retrieved 2008-11-16.
- ^ Glickman MS, Jacobs WR (2001). "Microbial pathogenesis of Mycobacterium tuberculosis: dawn of a discipline". Cell. 104 (4): 477–85. doi:10.1016/S0092-8674(01)00236-7. PMID 11239406.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Flowers T (1995). "Quarantining the noncompliant TB patient: catching the "Red Snapper"". Journal of health and hospital law : a publication of the American Academy of Hospital Attorneys of the American Hospital Association. 28 (2): 95–105. PMID 10141473.
- ^ Madigan M, Martinko J (editors) (2005). Brock Biology of Microorganisms (11th ed.). Prentice Hall. ISBN 0-13-144329-1.
{{cite book}}:|author=has generic name (help) - ^ Reddy JR, Kwang J, Lechtenberg KF, Khan NC, Prasad RB, Chengappa MM (2002). "An immunochromatographic serological assay for the diagnosis of Mycobacterium tuberculosis". Comp. Immunol. Microbiol. Infect. Dis. 25 (1): 21–7. doi:10.1016/S0147-9571(01)00016-9. PMID 11831744.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Robert Koch and Tuberculosis: Koch's Famous Lecture". Nobel Foundation. 2008. Retrieved 2008-11-18.
External links
- Database of Mycobacterium tuberculosis genome sequences and related information.
