Meloxicam: Difference between revisions
striked out tablet - Meloxicam tablets have not been approved by the FDA for use in dogs - |
|||
| Line 157: | Line 157: | ||
Under the brand name '''Metacam''', meloxicam is also used in the [[veterinary medicine|veterinary]] field, most commonly in dogs and cattle, but also in other animals such as cats and exotics; in the U.S. is indicated for management of pain and inflammation associated with [[osteoarthritis]] in dogs (FDA.gov), and in Europe, where the product has been available since the early 1990s, it is also prescribed and licensed for other anti-inflammatory benefits including relief from both [[acute pain|acute]] and [[chronic pain]] in dogs and cats. Side effects in animals are similar to those found in humans; the principal side effect is gastrointestinal irritation (vomiting, diarrhea and [[Peptic ulcer|ulceration]]). Rarer but important side effects include liver and kidney toxicity. |
Under the brand name '''Metacam''', meloxicam is also used in the [[veterinary medicine|veterinary]] field, most commonly in dogs and cattle, but also in other animals such as cats and exotics; in the U.S. is indicated for management of pain and inflammation associated with [[osteoarthritis]] in dogs (FDA.gov), and in Europe, where the product has been available since the early 1990s, it is also prescribed and licensed for other anti-inflammatory benefits including relief from both [[acute pain|acute]] and [[chronic pain]] in dogs and cats. Side effects in animals are similar to those found in humans; the principal side effect is gastrointestinal irritation (vomiting, diarrhea and [[Peptic ulcer|ulceration]]). Rarer but important side effects include liver and kidney toxicity. |
||
For many years, both [[injectable]] and [[Dosage forms#Oral dosage forms|oral]] ([[Suspension (chemistry)|liquid]] and tablet) formulations of meloxicam have been licensed for use in dogs, and injectable ones for use in cats. In June 2007, a new oral version of Metacam was licensed in [[Europe]] for the long-term relief of pain in cats. As of June 2008, Meloxicam is registered for long term use in cats in Australia, New Zealand, and throughout Europe. |
For many years, both [[injectable]] and [[Dosage forms#Oral dosage forms|oral]] ([[Suspension (chemistry)|liquid]] and <s>tablet</s>) formulations of meloxicam have been licensed for use in dogs, and injectable ones for use in cats. In June 2007, a new oral version of Metacam was licensed in [[Europe]] for the long-term relief of pain in cats. As of June 2008, Meloxicam is registered for long term use in cats in Australia, New Zealand, and throughout Europe. |
||
'''Meloxicam tablets have not been approved by the FDA for use in dogs''' due to the lack of safety and efficacy studies in the canine species. Metacam Oral Suspension is approved by the FDA to control pain and inflammation associated with osteoarthritis in dogs. If you or your veterinarian have any additional questions you may contact Boehringer Ingelheim Vetmedica, Inc Veterinary Technical Services at 866-638-2226. |
|||
==References== |
==References== |
||
Revision as of 20:15, 21 April 2010
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 89% |
| Protein binding | 99.4% |
| Metabolism | Hepatic (CYP2C9 and 3A4-mediated) |
| Elimination half-life | 15 to 20 hours |
| Excretion | Urine and faeces equally |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.113.257 |
| Chemical and physical data | |
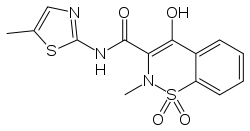
| Formula | C14H13N3O4S2 |
| Molar mass | 351.403 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| (verify) | |
Meloxicam is a nonsteroidal anti-inflammatory drug used as an analgesic, fever reducer and anti-inflammatory. It is a derivative of oxicam, closely related to piroxicam, and falls in the enolic acid group of NSAIDs.[2] It was developed by Boehringer-Ingelheim.
Availability
In Europe it is marketed under the brand names Movalis, Melox, and Recoxa. In the Philippines it is generally marketed as the brand name Moxen. In the UK, U.S., Middle East and Australia it is generally marketed under the brand name Mobic, in Germany as Mobec, and in Canada as Mobicox. In Latin America, the drug is marketed as Tenaron, Ilacox, Mavicam, or Melocam. A veterinary formulation of the drug is marketed as Metacam or Petcam.

Mechanism of action
Meloxicam inhibits cyclooxygenase (COX), the enzyme responsible for converting arachidonic acid into prostaglandin H2—the first step in the synthesis of prostaglandins, which are mediators of inflammation. Meloxicam has been shown, especially at its low therapeutic dose, selectively to inhibit COX-2 over COX-1.[3]
Meloxicam concentrations in synovial fluid range from 40% to 50% of those in plasma. The free fraction in synovial fluid is 2.5 times higher than in plasma, due to the lower albumin content in synovial fluid as compared to plasma. The significance of this penetration is unknown,[2] but it may account for the fact that it performs exceptionally well in treatment of arthritis in animal models.[4]
Adverse effects
Meloxicam use can result in gastrointestinal toxicity and bleeding, tinnitus, blinding headaches, rash, very dark or black stool (sign of intestinal bleeding). It has less gastrointestinal side effects than diclofenac,[5] piroxicam,[6] naproxen,[7] and perhaps all other NSAIDs which are not COX-2 selective.[5] Although meloxicam does inhibit thromboxane A, it does not appear to do so at levels that would interfere with platelet function.

Veterinary use
Under the brand name Metacam, meloxicam is also used in the veterinary field, most commonly in dogs and cattle, but also in other animals such as cats and exotics; in the U.S. is indicated for management of pain and inflammation associated with osteoarthritis in dogs (FDA.gov), and in Europe, where the product has been available since the early 1990s, it is also prescribed and licensed for other anti-inflammatory benefits including relief from both acute and chronic pain in dogs and cats. Side effects in animals are similar to those found in humans; the principal side effect is gastrointestinal irritation (vomiting, diarrhea and ulceration). Rarer but important side effects include liver and kidney toxicity.
For many years, both injectable and oral (liquid and tablet) formulations of meloxicam have been licensed for use in dogs, and injectable ones for use in cats. In June 2007, a new oral version of Metacam was licensed in Europe for the long-term relief of pain in cats. As of June 2008, Meloxicam is registered for long term use in cats in Australia, New Zealand, and throughout Europe.
Meloxicam tablets have not been approved by the FDA for use in dogs due to the lack of safety and efficacy studies in the canine species. Metacam Oral Suspension is approved by the FDA to control pain and inflammation associated with osteoarthritis in dogs. If you or your veterinarian have any additional questions you may contact Boehringer Ingelheim Vetmedica, Inc Veterinary Technical Services at 866-638-2226.
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b "Meloxicam official FDA information, side effects, and uses". Drugs.com. March 2010. Retrieved 17 March 2010.
- ^ Noble, S; Balfour, JA (1996). "Meloxicam". Drugs. 51 (3): 424–30, discussion 431-32. PMID 8882380.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Engelhardt, G; Homma, D; Schlegel, K; Utzmann, R; Schnitzler, C (1995). "Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new non-steroidal anti-inflammatory agent with favourable gastrointestinal tolerance". Inflammation Research. 44 (10): 423–433. doi:10.1007/BF01757699. PMID 8564518.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Hawkey, C; Kahan, A; Steinbrü, K; Alegre, C; Baumelou, E; Bégaud, B; Dequeker, J; Isomäki, H; Littlejohn, G; Mau, J; Papazoglous, S (the International Melissa Study Group) (1998). "Gastrointestinal tolerability of meloxicam compared to diclofenac in osteoarthritis patients". Rheumatology. 37 (9): 937-45(9).
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Dequeker, J; Hawkey, C; Kahan, A; Steinbruck, K; Alegre, C; Baumelou, E; Begaud, B; Isomaki, H; Littlejohn, G; Mau, J; Papazoglou, S (1998). "Improvement in gastrointestinal tolerability of the selective cyclooxygenase (COX)-2 inhibitor, meloxicam, compared with piroxicam: results of the Safety and Efficacy Large-scale Evaluation of COX- inhibiting Therapies (SELECT) trial in osteoarthritis". The British Journal of Rheumatology. 37: 946–51.
- ^ Wojtulewski, JA; Schattenkirchner, M; Barceló, P; Le Loët, X; Bevis, PJR; Bluhmki, E; Distel, M. "A Six-Month Double-Blind Trial to Compare the Efficacy and Safety of Meloxicam 7.5 mg Daily and Naproxen 750 mg Daily in Patients with Rheumatoid Arthritis". Rheumatology. 35, Supplement 1: 22–8.
