Autosomal dominant cerebellar ataxia
| Autosomal dominant cerebellar ataxia | |
|---|---|
| Other names | Autosomal dominant spinocerebellar ataxia[1] |
 | |
| Autosomal dominant is the manner in which this condition is inherited | |
| Symptoms | Multi system involvement[2] |
| Types | ADCS type1, ADCA type 2, ADCA type 3[2] |
| Diagnostic method | MRI, CT scan[3] |
| Treatment | Anticonvulsants may be used[2] |
Autosomal dominant cerebellar ataxia (ADCA) is a form of spinocerebellar ataxia inherited in an autosomal dominant manner. ADCA is a genetically inherited condition that causes deterioration of the nervous system leading to disorder and a decrease or loss of function to regions of the body.[2]
Degeneration occurs at the cellular level and in certain subtypes results in cellular death. Cellular death or dysfunction causes a break or faulty signal in the line of communication from the central nervous system to target muscles in the body. When there is impaired communication or a lack of communication entirely, the muscles in the body do not function correctly. Muscle control complications can be observed in multiple balance, speech, and motor or movement impairment symptoms. ADCA is divided into three types and further subdivided into subtypes known as SCAs (spinocerebellar ataxias).[4]
Types
[edit]Currently there are 27 subtypes have been identified: SCA1-SCA4, SCA8, SCA10, SCA12-SCA14, SCA15/SCA16, SCA17-SCA23, SCA25, SCA27, SCA28, SCA32, SCA34-SCA37, autosomal dominant cerebellar ataxia and dentatorubral pallidoluysian atrophy.[3]
Type 1
[edit]Type I ADCA is characterized by different symptoms of ataxia as well as other conditions that are dependent on the subtype. Type 1 ADCA is divided into 3 subclasses based on pathogenesis of the subtypes each contain.[4][3][5]

- Subtype 1 – subtypes in the first subclass are caused by CAG nucleotide repeats in the DNA, which code for the amino acid glutamine. This glutamine is toxic to the cell on the level of proteins and has degenerative effects. Within the first subclass of Type 1 are SCA1, SCA2, SCA3, SCA17, and DRPLA. This first subclass is the most common of Type 1 ADCAs with SCA3 being the most common subtype of all of Type 1. SCA3, Machado-Joseph disease, is the most common because the mutation repeats more than 56 times while the regular length is around 13 to 31.[4]
- Subtype 2 – the second subclass of Type 1 ADCA is also caused by the same nucleotide repeats but instead in RNA and in a region that does not code for proteins. Gene expression is affected instead of proteins in subtype two SCAs because of this. Subtype 2 contains SCA8, SCA10, and SCA12.[4]
- Subtype 3 – the third subclass of Type 1 ADCA is caused by different mutations and deletions in genes. It comprises SCA13, SCA14, SCA15, SCA16, SCA27, and SCA28.[4]
Type 2/3
[edit]Type II ADCA is composed of SCA7 and syndromes associated with pigmentary maculopathies.[4] SCA7 is a disease that specifically displays retinal degeneration, along with the common degeneration of the cerebellum. Moving further into SCA7's pathology, a similar genetic process is described, while the function of ATXN7 (an ataxin gene) is much like a component of the SAGA complex. The SAGA complex uses two histone-modifying techniques to regulate transcription. These activities are the Gcn5 histone acetyltransferase and the Usp22 deubiquitinase. Mutant ATXN7 in HAT activity causes an increase in activity, which was reported from an in-vivo analysis in the retina. There are also studies that show a loss in activity when human ATXN7 in yeast was used. The SCA7 autosomal-dominant inheritance pattern is similar to a mutant ATXN5-induced gain in Gcn5 HAT.[6] Spinocerebellar ataxia type 15 has been classified as an ADCA Type III as it has been noted to have postural and action tremor in addition to cerebellar ataxia.[4] Additionally, spinocerebellar ataxia type 20 (SCA20) is organized in ADCA III that often exhibits disease-like symptoms at an earlier age, sometime starting at fourteen years old.[4][7][8]
Symptoms and signs
[edit]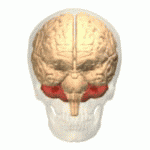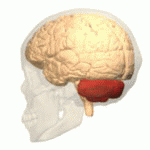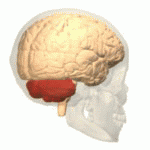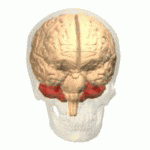
Symptoms typically are onset in the adult years, although, childhood cases have also been observed. Common symptoms include a loss of coordination which is often seen in walking, and slurred speech. ADCA primarily affects the cerebellum, as well as, the spinal cord.[9] Some signs and symptoms are:[2]
- Episodes of altered level of consciousness
- Neurological regression
- Multi-system involvement
- Movement disorders.
- Cerebellar dysfunction
Genetics
[edit]In terms of the genetics of autosomal dominant cerebellar ataxia 11 of 18 known genes are caused by repeated expansions in corresponding proteins, sharing the same mutational mechanism. SCAs can be caused by conventional mutations or large rearrangements in genes that make glutamate and calcium signaling, channel function, tau regulation and mitochondrial activity or RNA alteration.[10]
The mechanism of Type I is not completely known, however, Whaley, et al. suggest the polyglutamine product is toxic to the cell at a protein level, this effect may be done by transcriptional dysregulation and disruption of calcium homeostasis which causes apoptosis to occur earlier.[4]
Diagnosis
[edit]In diagnosing autosomal dominant cerebellar ataxia the individuals clinical history or their past health examinations, a current physical examination to check for any physical abnormalities, and a genetic screening of the patients genes and the genealogy of the family are done.[11] The large category of cerebellar ataxia is caused by a deterioration of neurons in the cerebellum, therefore magnetic resonance imaging (MRI) is used to detect any structural abnormality such as lesions which are the primary cause of the ataxia. Computed tomography (CT) scans can also be used to view neuronal deterioration, but the MRI provides a more accurate and detailed picture.[12]
Treatments
[edit]In terms of a cure there is currently none available, however for the disease to manifest itself, it requires mutant gene expression.[6] Manipulating the use of protein homeostasis regulators can be therapeutic agents, or a treatment to try and correct an altered function that makes up the pathology is one current idea put forth by Bushart, et al.[6][13] There is some evidence that for SCA1 and two other polyQ disorders that the pathology can be reversed after the disease is underway.[6]
Epidemiology
[edit]In terms of frequency, it is estimated at 2 per 100,000, it has identified in different regions of the world. Some clusters of certain types of autosomal dominant cerebellar ataxia reach a prevalence of 5 per 100,000.[2]
References
[edit]- ^ RESERVED, INSERM US14-- ALL RIGHTS. "Orphanet: Autosomal dominant cerebellar ataxia". www.orpha.net. Archived from the original on 31 August 2019. Retrieved 8 August 2019.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ^ a b c d e f "Autosomal Dominant Cerebellar Ataxia information page. Patient | Patient". Patient. Archived from the original on 2021-08-13. Retrieved 2016-03-25.
- ^ a b c RESERVED, INSERM US14 -- ALL RIGHTS. "Orphanet: Autosomal dominant cerebellar ataxia type 1". www.orpha.net. Archived from the original on 2020-11-12. Retrieved 2016-03-25.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ^ a b c d e f g h i Whaley, Nathaniel; Fujioka, Shinsuke; Wszolek, Zbigniew K (1 January 2011). "Autosomal dominant cerebellar ataxia type I: A review of the phenotypic and genotypic characteristics". Orphanet Journal of Rare Diseases. 6 (1): 33. doi:10.1186/1750-1172-6-33. PMC 3123548. PMID 21619691.
- ^ "SCA1". Genetics Home Reference. 2016-03-21. Archived from the original on 2021-04-17. Retrieved 2016-03-25.
- ^ a b c d Orr, H. T. (16 April 2012). "The cell biology of disease: Cell biology of spinocerebellar ataxia". The Journal of Cell Biology. 197 (2): 167–177. doi:10.1083/jcb.201105092. PMC 3328388. PMID 22508507.
- ^ Pulst, Stefan M. (1993-01-01). Pagon, Roberta A.; Adam, Margaret P.; Ardinger, Holly H.; Wallace, Stephanie E.; Amemiya, Anne; Bean, Lora J.H.; Bird, Thomas D.; Fong, Chin-To; Mefford, Heather C. (eds.). Spinocerebellar Ataxia Type 2. Seattle (WA): University of Washington, Seattle. PMID 20301452. Archived from the original on 2017-01-18. Retrieved 2017-08-30.Revised 2015
- ^ Fujioka, Shinsuke; Sundal, Christina; Wszolek, Zbigniew K (2013-01-18). "Autosomal dominant cerebellar ataxia type III: a review of the phenotypic and genotypic characteristics". Orphanet Journal of Rare Diseases. 8 (1): 14. doi:10.1186/1750-1172-8-14. PMC 3558377. PMID 23331413.
- ^ Bird, Thomas D. (1993-01-01). Pagon, Roberta A.; Adam, Margaret P.; Ardinger, Holly H.; Wallace, Stephanie E.; Amemiya, Anne; Bean, Lora J.H.; Bird, Thomas D.; Fong, Chin-To; Mefford, Heather C. (eds.). Hereditary Ataxia Overview. Seattle (WA): University of Washington, Seattle. PMID 20301317. Archived from the original on 2021-10-17. Retrieved 2017-08-30.Last Revision: March 3, 2016.
- ^ Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Durr A. doi:10.1016/S1474-4422(10)70183-6 – via ScienceDirect (Subscription may be required or content may be available in libraries.)
- ^ Brusse E, Maat-Kievit JA, van Swieten JC (2007). "Diagnosis and management of early- and late-onset cerebellar ataxia". Clin. Genet. 71 (1): 12–24. doi:10.1111/j.1399-0004.2006.00722.x. PMID 17204042. S2CID 25779423.
- ^ Ludger, Schols (2003). "Autosomal Dominant Cerebellar Ataxia" (PDF). Orphanet. Archived from the original (PDF) on 30 August 2017. Retrieved 25 March 2016.
- ^ Bushart, David D.; Murphy, Geoffrey G.; Shakkottai, Vikram G. (2016-01-01). "Precision medicine in spinocerebellar ataxias: treatment based on common mechanisms of disease". Annals of Translational Medicine. 4 (2): 25. doi:10.3978/j.issn.2305-5839.2016.01.06. ISSN 2305-5839. PMC 4731605. PMID 26889478.
Further reading
[edit]- Burk, K (1996). "Autosomal dominant cerebellar ataxia type I Clinical features and MRI in families with SCA1, SCA2 and SCA". Brain. 119 (5): 1497–1505. doi:10.1093/brain/119.5.1497. PMID 8931575.
- Louis, Elan D.; Mayer, Stephan A.; Rowland, Lewis P. (2015-08-31). Merritt's Neurology. Lippincott Williams & Wilkins. ISBN 9781496321077.
- LeDoux, Mark S. (2005-01-25). Movement Disorders: Genetics and Models. Academic Press. ISBN 9780080470566.
