Myelin oligodendrocyte glycoprotein
| MOG | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | MOG, BTN6, BTNL11, MOGIG2, NRCLP7, myelin oligodendrocyte glycoprotein | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 159465; MGI: 97435; HomoloGene: 111009; GeneCards: MOG; OMA:MOG - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Myelin oligodendrocyte glycoprotein (MOG) is a glycoprotein believed to be important in the myelination of nerves in the central nervous system (CNS). In humans this protein is encoded by the MOG gene.[5][6][7] It is speculated to serve as a necessary "adhesion molecule" to provide structural integrity to the myelin sheath and is known to develop late on the oligodendrocyte.[8]
Molecular function
[edit]While the primary molecular function of MOG is not yet known, its likely role with the myelin sheath is either in sheath "completion and/or maintenance".[7] More specifically, MOG is speculated to be "necessary" as an "adhesion molecule" on the myelin sheath of the CNS to provide the structural integrity of the myelin sheath.[8]"
MOG's cDNA coding region in humans have been shown to be "highly homologous"[9] to rats, mice, and bovine, and hence highly conserved. This suggests "an important biological role for this protein".[7]
Physiology
[edit]The gene for MOG, found on chromosome 6 p21.3-p22,[10] was first sequenced in 1995.[3] It is a transmembrane protein expressed on the surface of oligodendrocyte cell and on the outermost surface of myelin sheaths. "MOG is a quantitatively minor type I transmembrane protein,[11] and is found exclusively in the CNS. "A single Ig-domain is exposed to the extracellular space"[11] and consequently allows autoantibodies easy access. and therefore is easily accessible to autoantibodies too.[7][11] The MOG "primary nuclear transcript … is 15,561 nucleotides in length"[7] and, for humans, it has eight exons which are "separated by seven introns".[7] The introns "contain numerous reptitive [sic] DNA[7]" sequences, among which are "14 Alu sequences within 3 introns",[7] and have a range varying from 242 to 6484 bp.
Structure
[edit]Alternatively spliced human mRNA of the MOG gene form at least nine isoforms.[12]
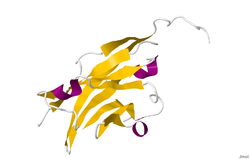
The crystal structure of myelin oligodendrocyte glycoprotein was determined by x-ray diffraction at a resolution of 1.45 Angstroms, using protein from the Norway rat. This protein is 139 residues long, and is a member of the immunoglobulin superfamily.[13] The dssp secondary structure of the protein is 6% helical and 43% beta sheet: there are three short helical segments and ten beta strands.[14] The beta strands are within two antiparallel beta sheets that form an immunoglobulin-like beta-sandwich fold.[15] Several features of the protein structure suggest MOG has a role as an "adhesin in the completion and/or compaction of the myelin sheath." There is a "significant strip" of electronegative charge beginning near the N-terminus and running about half the length of the molecule. Also, MOG was shown to dimerize in solution, and the shape complementarity index is high at the dimer interface, suggesting a "biologically relevant MOG dimer."[16]
Synthesis
[edit]Developmentally, MOG is formed "very late on oligodendrocytes and the myelin sheath".[8]
Role in disease
[edit]Non-inflammatory demyelinating diseases
[edit]Interest in MOG has centered on its role in demyelinating diseases. Some of them are not-inflammatory, such as adrenoleukodystrophy, vanishing white matter disease, and Rubella induced mental retardation.[17]
Anti-MOG associated inflammatory demyelinating diseases
[edit]MOG has received much of its laboratory attention in studies dealing with MS. Several studies have shown a role for antibodies against MOG in the pathogenesis of MS,[8][18] though most of them were written before the discovery of NMO-IgG and the NMO spectrum of diseases.
Anti-MOG status is different depending whether it is measured by ELISA or by microarray (CBA). The proper way to identify it is by microarray, reacting patient serum with living cells, and detecting the binding IgG via a fluorescent-labeled secondary antibody.[19]
In animal models
[edit]Animal models of MS, namely Experimental Autoimmune Encephalomyelitis (EAE) models, have shown that "MOG-specific EAE models (of different animal strains) display/mirror human multiple sclerosis",[8] but basically explains the part involved in the optic neuritis.[20] These models with anti-MOG antibodies have been investigated extensively and are considered the only antibodies with demyelinating capacity[8] but again, EAE pathology is closer to NMO and ADEM than to the confluent demyelination observed in MS.
Anti-MOG antibodies have been shown to behave similarly to AQP4 antibodies in animal models,[20] and are considered a biomarker against the MS diagnosis[21][22]
In seronegative neuromyelitis optica
[edit]Anti-MOG autoimmunity has been found to be involved in most AQP4-seronegative NMO[23][24] and also in optic neuritis and some fulminant forms of ADEM.[25] MOG antibodies in NMOSD are variable depending on the seropositivity status.[26]
In other conditions
[edit]The presence of anti-MOG autoantibodies has been associated with the following conditions[27]
- Most cases of aquaporin-4-seronegative neuromyelitis optica: NMO derived from an antiMOG associated encephalomyelitis,[28]
- Some cases of acute disseminated encephalomyelitis, specially the recurrent ones (MDEM)[29] and the fulminant courses[25]
- Some cases of multiple sclerosis[27]
- isolated optic neuritis or transverse myelitis[27]
References
[edit]- ^ a b c ENSG00000232641, ENSG00000137345, ENSG00000230885, ENSG00000236561, ENSG00000237834, ENSG00000204655, ENSG00000234623 GRCh38: Ensembl release 89: ENSG00000234096, ENSG00000232641, ENSG00000137345, ENSG00000230885, ENSG00000236561, ENSG00000237834, ENSG00000204655, ENSG00000234623 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000076439 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Pham-Dinh D, Della Gaspera B, Kerlero de Rosbo N, Dautigny A (September 1995). "Structure of the human myelin/oligodendrocyte glycoprotein gene and multiple alternative spliced isoforms". Genomics. 29 (2): 345–52. doi:10.1006/geno.1995.9995. PMID 8666381.
- ^ Pham-Dinh D, Jones EP, Pitiot G, Della Gaspera B, Daubas P, Mallet J, Le Paslier D, Fischer Lindahl K, Dautigny A (1995). "Physical mapping of the human and mouse MOG gene at the distal end of the MHC class Ib region". Immunogenetics. 42 (5): 386–91. doi:10.1007/bf00179400. PMID 7590972. S2CID 8310478.
- ^ a b c d e f g h Roth MP, Malfroy L, Offer C, Sevin J, Enault G, Borot N, Pontarotti P, Coppin H (July 1995). "The human myelin oligodendrocyte glycoprotein (MOG) gene: complete nucleotide sequence and structural characterization". Genomics. 28 (2): 241–50. doi:10.1006/geno.1995.1137. PMID 8530032.
- ^ a b c d e f Berger, T., Innsbruck Medical University Dept. of Neurology interviewed by S. Gillooly, Nov. 24, 2008.
- ^ Pham-Dinh D, Allinquant B, Ruberg M, Della Gaspera B, Nussbaum JL, Dautigny A (December 1994). "Characterization and expression of the cDNA coding for the human myelin/oligodendrocyte glycoprotein". Journal of Neurochemistry. 63 (6): 2353–6. doi:10.1046/j.1471-4159.1994.63062353.x. PMID 7964757. S2CID 2788720.
- ^ Pham-Dinh D, Mattei MG, Nussbaum JL, Roussel G, Pontarotti P, Roeckel N, Mather IH, Artzt K, Lindahl KF, Dautigny A (September 1993). "Myelin/oligodendrocyte glycoprotein is a member of a subset of the immunoglobulin superfamily encoded within the major histocompatibility complex". Proceedings of the National Academy of Sciences of the United States of America. 90 (17): 7990–4. Bibcode:1993PNAS...90.7990P. doi:10.1073/pnas.90.17.7990. PMC 47273. PMID 8367453.
- ^ a b c Berger T, Reindl M (August 2007). "Multiple sclerosis: disease biomarkers as indicated by pathophysiology". Journal of the Neurological Sciences. 259 (1–2): 21–6. doi:10.1016/j.jns.2006.05.070. PMID 17367811. S2CID 23257594.
- ^ Boyle LH, Traherne JA, Plotnek G, Ward R, Trowsdale J (September 2007). "Splice variation in the cytoplasmic domains of myelin oligodendrocyte glycoprotein affects its cellular localisation and transport". Journal of Neurochemistry. 102 (6): 1853–62. doi:10.1111/j.1471-4159.2007.04687.x. PMC 2156149. PMID 17573820.
- ^ Breithaupt C, Schubart A, Zander H, Skerra A, Huber R, Linington C, Jacob U (August 2003). "Structural insights into the antigenicity of myelin oligodendrocyte glycoprotein". Proceedings of the National Academy of Sciences of the United States of America. 100 (16): 9446–51. Bibcode:2003PNAS..100.9446B. doi:10.1073/pnas.1133443100. PMC 170938. PMID 12874380.
- ^ Kabsch W, Sander C (December 1983). "Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features". Biopolymers. 22 (12): 2577–637. doi:10.1002/bip.360221211. PMID 6667333. S2CID 29185760.
- ^ Murzin AG, Brenner SE, Hubbard T, Chothia C (April 1995). "SCOP: a structural classification of proteins database for the investigation of sequences and structures". Journal of Molecular Biology. 247 (4): 536–40. doi:10.1016/S0022-2836(05)80134-2. PMID 7723011.
- ^ Clements CS, Reid HH, Beddoe T, Tynan FE, Perugini MA, Johns TG, Bernard CC, Rossjohn J (September 2003). "The crystal structure of myelin oligodendrocyte glycoprotein, a key autoantigen in multiple sclerosis". Proceedings of the National Academy of Sciences of the United States of America. 100 (19): 11059–64. Bibcode:2003PNAS..10011059C. doi:10.1073/pnas.1833158100. PMC 196926. PMID 12960396.
- ^ Cong H, Jiang Y, Tien P (November 2011). "Identification of the myelin oligodendrocyte glycoprotein as a cellular receptor for rubella virus". Journal of Virology. 85 (21): 11038–47. doi:10.1128/JVI.05398-11. PMC 3194935. PMID 21880773.
- ^ Berger T, Rubner P, Schautzer F, Egg R, Ulmer H, Mayringer I, Dilitz E, Deisenhammer F, Reindl M (July 2003). "Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event". The New England Journal of Medicine. 349 (2): 139–45. doi:10.1056/NEJMoa022328. PMID 12853586.
- ^ Ichiro Nakashima, Anti-myelin oligodendrocyte glycoprotein antibody in demyelinating diseases [1]
- ^ a b Kezuka T, Usui Y, Yamakawa N, Matsunaga Y, Matsuda R, Masuda M, Utsumi H, Tanaka K, Goto H (June 2012). "Relationship between NMO-antibody and anti-MOG antibody in optic neuritis". Journal of Neuro-Ophthalmology. 32 (2): 107–10. doi:10.1097/WNO.0b013e31823c9b6c. PMID 22157536. S2CID 46667141.
- ^ Ketelslegers IA, Van Pelt DE, Bryde S, Neuteboom RF, Catsman-Berrevoets CE, Hamann D, Hintzen RQ (October 2015). "Anti-MOG antibodies plead against MS diagnosis in an Acquired Demyelinating Syndromes cohort". Multiple Sclerosis. 21 (12): 1513–20. doi:10.1177/1352458514566666. PMID 25662345. S2CID 25321614.
- ^ Kitley J, Woodhall M, Waters P, Leite MI, Devenney E, Craig J, Palace J, Vincent A (September 2012). "Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype". Neurology. 79 (12): 1273–7. doi:10.1212/WNL.0b013e31826aac4e. PMID 22914827. S2CID 855313.
- ^ Pröbstel AK, Rudolf G, Dornmair K, Collongues N, Chanson JB, Sanderson NS, Lindberg RL, Kappos L, de Seze J, Derfuss T (2015). "Anti-MOG antibodies are present in a subgroup of patients with a neuromyelitis optica phenotype". Journal of Neuroinflammation. 12 (1): 46. doi:10.1186/s12974-015-0256-1. PMC 4359547. PMID 25889963.
- ^ CYNTHIA MCKELVEY, Press Report, What's the Role of Myelin Oligodendrocyte Glycoprotein in NMO? [2]
- ^ a b Di Pauli F, Höftberger R, Reindl M, Beer R, Rhomberg P, Schanda K, Sato D, Fujihara K, Lassmann H, Schmutzhard E, Berger T (December 2015). "Fulminant demyelinating encephalomyelitis: Insights from antibody studies and neuropathology". Neurology: Neuroimmunology & Neuroinflammation. 2 (6): e175. doi:10.1212/NXI.0000000000000175. PMC 4635550. PMID 26587556.
- ^ Berger T, Reindl M (August 2015). "Antibody biomarkers in CNS demyelinating diseases - a long and winding road". European Journal of Neurology. 22 (8): 1162–8. doi:10.1111/ene.12759. PMID 26010364. S2CID 39301229.
- ^ a b c Reindl M, Di Pauli F, Rostásy K, Berger T (August 2013). "The spectrum of MOG autoantibody-associated demyelinating diseases". Nature Reviews. Neurology. 9 (8): 455–61. doi:10.1038/nrneurol.2013.118. PMID 23797245. S2CID 7219279.
- ^ Spadaro M, Gerdes LA, Mayer MC, Ertl-Wagner B, Laurent S, Krumbholz M, Breithaupt C, Högen T, Straube A, Giese A, Hohlfeld R, Lassmann H, Meinl E, Kümpfel T (March 2015). "Histopathology and clinical course of MOG-antibody-associated encephalomyelitis". Annals of Clinical and Translational Neurology. 2 (3): 295–301. doi:10.1002/acn3.164. PMC 4369279. PMID 25815356.
- ^ Baumann M, Hennes EM, Schanda K, Karenfort M, Kornek B, Seidl R, Diepold K, Lauffer H, Marquardt I, Strautmanis J, Syrbe S, Vieker S, Höftberger R, Reindl M, Rostásy K (2016). "Children with multiphasic disseminated encephalomyelitis and antibodies to the myelin oligodendrocyte glycoprotein (MOG): Extending the spectrum of MOG antibody positive diseases". Multiple Sclerosis. 22 (14): 1821–1829. doi:10.1177/1352458516631038. PMID 26869530. S2CID 30428892.





