Lysosome: Difference between revisions
ClueBot NG (talk | contribs) m Reverting possible vandalism by 206.123.212.121 to version by 96.49.178.195. False positive? Report it. Thanks, ClueBot NG. (1432941) (Bot) |
|||
| Line 18: | Line 18: | ||
== Lysosomotropism == |
== Lysosomotropism == |
||
Weak [[Base (chemistry)|bases]] with [[Lipophilicity|lipophilic]] properties accumulate in acidic intracellular compartments like lysosomes. While the plasma and lysosomal membranes are permeable for neutral and uncharged species of weak bases, the charged protonated species of weak bases do not permeate biomembranes and accumulate within lysosomes. The concentration within lysosomes may reach levels 100 to 1000 fold higher than extracellular concentrations. This phenomenon is called "lysosomotropism"<ref>de Duve C, de Barsy T, Poole B, Trouet A, Tulkens P, van Hoof F. Lysosomotropic agents. Biochem.Pharmacol. 23:2495-2531, 1974. PMID 4606365</ref> or "acid trapping". The amount of accumulation of lysosomotropic compounds may be estimated using a cell based mathematical model.<ref>Trapp S, Rosania G, Horobin RW, Kornhuber J. Quantitative modeling of selective lysosomal targeting for drug design. Eur.Biophys.J. 37 (8):1317-1328, 2008. PMID 18504571</ref> |
Weak [[Base (chemistry)|bases]] with [[Lipophilicity|lipophilic]] properties accumulate in acidic intracellular compartments like lysosomes. While the plasma and lysosomal membranes are permeable for neutral and uncharged species of weak bases, the charged protonated species of weak bases do not permeate biomembranes and accumulate within lysosomes.aubrey smells The concentration within lysosomes may reach levels 100 to 1000 fold higher than extracellular concentrations. This phenomenon is called "lysosomotropism"<ref>de Duve C, de Barsy T, Poole B, Trouet A, Tulkens P, van Hoof F. Lysosomotropic agents. Biochem.Pharmacol. 23:2495-2531, 1974. PMID 4606365</ref> or "acid trapping". The amount of accumulation of lysosomotropic compounds may be estimated using a cell based mathematical model.<ref>Trapp S, Rosania G, Horobin RW, Kornhuber J. Quantitative modeling of selective lysosomal targeting for drug design. Eur.Biophys.J. 37 (8):1317-1328, 2008. PMID 18504571</ref> |
||
A significant part of the clinically approved drugs are lipophilic weak bases with lysosomotropic properties. This explains a number of pharmacological properties of these drugs, such as high tissue-to-blood concentration gradients or long tissue elimination half-lifes; these properties have been found for drugs such as [[haloperidol]],<ref>Kornhuber J, Schultz A, Wiltfang J, Meineke I, Gleiter CH, Zöchling R, Boissl KW, Leblhuber F, Riederer P. Persistence of haloperidol in human brain tissue. Am.J.Psychiatry 156:885-890, 1999. PMID 10360127</ref> [[levomepromazine]] <ref>Kornhuber J, Weigmann H, Röhrich J, Wiltfang J, Bleich S, Meineke I, Zöchling R, Hartter S, Riederer P, Hiemke C. Region specific distribution of levomepromazine in the human brain. J.Neural Transm. 113:387-397, 2006. PMID 15997416</ref> and [[amantadine]].<ref>Kornhuber J, Quack G, Danysz W, Jellinger K, Danielczyk W, Gsell W, Riederer P. Therapeutic brain concentration of the NMDA receptor antagonist amantadine. Neuropharmacology 34:713-721, 1995. PMID 8532138</ref> However, in addition to high tissue concentrations and long elimination half-life is explained also by lipophilicity and absorption of drugs to fatty tissue structures. Important lysosomal enzymes, such as acid sphingomyelinase, may be inhibited by lysososomally accumulated drugs.<ref>Kornhuber J, Tripal P, Reichel M, Terfloth L, Bleich S, Wiltfang J, Gulbins E. Identification of new functional inhibitors of acid sphingomyelinase using a structure-property-activity relation model. J.Med.Chem. 51:219-237, 2008. PMID 18027916</ref><ref>Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, Mühle C, Terfloth L, Groemer TW, Spitzer GM, Liedl KR, Gulbins E, Tripal P. Identification of novel functional inhibitors of acid sphingomyelinase. PLoS ONE 6 (8):e23852, 2011. PMID 21909365</ref> Such compounds are termed FIASMAs (functional inhibitor of acid sphingomyelinase)<ref>Kornhuber J, Tripal P, Reichel M, Mühle C, Rhein C, Muehlbacher M, Groemer TW, Gulbins E. Functional inhibitors of acid sphingomyelinase (FIASMAs): a novel pharmacological group of drugs with broad clinical applications. Cell.Physiol.Biochem. 26:9-20, 2010. PMID 20502000</ref> and include for example [[fluoxetine]], [[sertraline]] or [[amitriptyline]]. |
A significant part of the clinically approved drugs are lipophilic weak bases with lysosomotropic properties. This explains a number of pharmacological properties of these drugs, such as high tissue-to-blood concentration gradients or long tissue elimination half-lifes; these properties have been found for drugs such as [[haloperidol]],<ref>Kornhuber J, Schultz A, Wiltfang J, Meineke I, Gleiter CH, Zöchling R, Boissl KW, Leblhuber F, Riederer P. Persistence of haloperidol in human brain tissue. Am.J.Psychiatry 156:885-890, 1999. PMID 10360127</ref> [[levomepromazine]] <ref>Kornhuber J, Weigmann H, Röhrich J, Wiltfang J, Bleich S, Meineke I, Zöchling R, Hartter S, Riederer P, Hiemke C. Region specific distribution of levomepromazine in the human brain. J.Neural Transm. 113:387-397, 2006. PMID 15997416</ref> and [[amantadine]].<ref>Kornhuber J, Quack G, Danysz W, Jellinger K, Danielczyk W, Gsell W, Riederer P. Therapeutic brain concentration of the NMDA receptor antagonist amantadine. Neuropharmacology 34:713-721, 1995. PMID 8532138</ref> However, in addition to high tissue concentrations and long elimination half-life is explained also by lipophilicity and absorption of drugs to fatty tissue structures. Important lysosomal enzymes, such as acid sphingomyelinase, may be inhibited by lysososomally accumulated drugs.<ref>Kornhuber J, Tripal P, Reichel M, Terfloth L, Bleich S, Wiltfang J, Gulbins E. Identification of new functional inhibitors of acid sphingomyelinase using a structure-property-activity relation model. J.Med.Chem. 51:219-237, 2008. PMID 18027916</ref><ref>Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, Mühle C, Terfloth L, Groemer TW, Spitzer GM, Liedl KR, Gulbins E, Tripal P. Identification of novel functional inhibitors of acid sphingomyelinase. PLoS ONE 6 (8):e23852, 2011. PMID 21909365</ref> Such compounds are termed FIASMAs (functional inhibitor of acid sphingomyelinase)<ref>Kornhuber J, Tripal P, Reichel M, Mühle C, Rhein C, Muehlbacher M, Groemer TW, Gulbins E. Functional inhibitors of acid sphingomyelinase (FIASMAs): a novel pharmacological group of drugs with broad clinical applications. Cell.Physiol.Biochem. 26:9-20, 2010. PMID 20502000</ref> and include for example [[fluoxetine]], [[sertraline]] or [[amitriptyline]]. |
||
Revision as of 20:08, 8 January 2013
| Cell biology | |
|---|---|
| Animal cell diagram | |
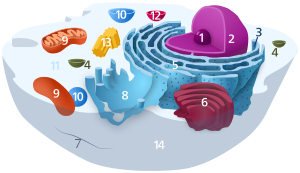 Components of a typical animal cell:
|
Lysosomes are celluler organelles that contain acid hydrolase enzymes that break down waste materials and cellular debris. They can be described as the stomach of the cell. They are found in animal cells, while their existence in yeasts and plants are disputed. Some biologists say the same roles are performed by lytic vacuoles,[1] while others suggest there is strong evidence that lysosomes are indeed in some plant cells.[2] Lysosomes digest excess or worn-out organelles, food particles, and engulf viruses or bacteria. The membrane around a lysosome allows the digestive enzymes to work at the 5 pH they require. Lysosomes fuse with autophagic vacuoles and dispense their enzymes into the autophagic vacuoles, digesting their contents. The name lysosome derives from the Greek words lysis, to separate, and soma, body. They are frequently nicknamed "suicide-bags" or "suicide-sacs" by cell biologists due to their autolysis. Lysosomes were discovered by the Belgian cytologist Christian de Duve in 1949.
The size of a lysosome varies from 0.1–1.2 μm.[3] At pH 4.8, the interior of the lysosomes is acidic compared to the slightly basic cytosol (pH 7.2). The lysosome maintains this pH differential by pumping protons (H+ ions) from the cytosol across the membrane via proton pumps and chloride ion channels. The lysosomal membrane protects the cytosol, and therefore the rest of the cell, from the degradative enzymes within the lysosome. The cell is additionally protected from any lysosomal acid hydrolases that drain into the cytosol, as these enzymes are pH-sensitive and do not function well or at all in the alkaline environment of the cytosol.This ensures that cytosolic molecules and organelles are not lysed in case there is leakage of the hydrolytic enzymes from the lysosome.
Lysosomes are the cell's waste disposal system and can digest some compounds. They are used for the digestion of macromolecules from phagocytosis (ingestion of other dying cells or larger extracellular material, like foreign invading microbes), endocytosis (where receptor proteins are recycled from the cell surface), and autophagy (wherein old or unneeded organelles or proteins, or microbes that have invaded the cytoplasm are delivered to the lysosome).
Other functions include digesting foreign bacteria (or other forms of waste) that invade a cell and helping repair damage to the plasma membrane by serving as a membrane patch, sealing the wound. In the past, lysosomes were thought to kill cells that are no longer wanted, such as those in the tails of tadpoles or in the web from the fingers of a 3- to 6-month-old fetus.
Formation
Many components of the animal cell can be seen recycled by using and sharing their membranous units . For instance, in endocytosis, a portion of the cell’s plasma membrane pinches off to form a vesicle that will eventually fuse with an organelle within the cell. Without active replenishment, the plasma membrane would continuously decrease in size. It is thought that lysosomes participate in this dynamic membrane exchange system and are formed by a gradual maturation process from endosomes.[4]
The production and transport of lysosomal proteins suggest one method of lysosome sustainment. Lysosomal protein genes are transcribed in the nucleus. mRNA transcripts exit the nucleus into the cytosol, where they are translated by ribosomes. The nascent peptide chains are translocated into the rough endoplasmic reticulum, where they are modified. Upon exiting the endoplasmic reticulum and entering the Golgi apparatus via vesicular transport, a specific lysosomal tag, mannose 6-phosphate, is added to the peptides. The presence of these tags allow for binding to mannose 6-phosphate receptors in the golgi apparatus, a phenomenon that is crucial for proper packaging into vesicles destined for the lysosomal system.[5] please give this note in sinhala
Upon leaving the golgi apparatus, the lysosomal enzyme-filled vesicle fuses with a late endosome, a relatively acidic organelle with an approximate pH of 5.5.[5] This acidic environment causes dissociation of the lysosomal enzymes from the mannose 6-phosphate receptors[5]. The enzymes are packed into vesicles for further transport to established lysosomes.[5] The late endosome itself can eventually mature into a mature lysosomes, as evidenced by the transport of endosomal membrane components from the lysosomes back to the endosomes.[4]
Lysosomotropism
Weak bases with lipophilic properties accumulate in acidic intracellular compartments like lysosomes. While the plasma and lysosomal membranes are permeable for neutral and uncharged species of weak bases, the charged protonated species of weak bases do not permeate biomembranes and accumulate within lysosomes.aubrey smells The concentration within lysosomes may reach levels 100 to 1000 fold higher than extracellular concentrations. This phenomenon is called "lysosomotropism"[6] or "acid trapping". The amount of accumulation of lysosomotropic compounds may be estimated using a cell based mathematical model.[7]
A significant part of the clinically approved drugs are lipophilic weak bases with lysosomotropic properties. This explains a number of pharmacological properties of these drugs, such as high tissue-to-blood concentration gradients or long tissue elimination half-lifes; these properties have been found for drugs such as haloperidol,[8] levomepromazine [9] and amantadine.[10] However, in addition to high tissue concentrations and long elimination half-life is explained also by lipophilicity and absorption of drugs to fatty tissue structures. Important lysosomal enzymes, such as acid sphingomyelinase, may be inhibited by lysososomally accumulated drugs.[11][12] Such compounds are termed FIASMAs (functional inhibitor of acid sphingomyelinase)[13] and include for example fluoxetine, sertraline or amitriptyline.
References
- ^ Samaj J, Read ND, Volkmann D, Menzel D, Baluska F (2005). "The endocytic network in plants". Trends Cell Biol. 15 (8): 425–33. doi:10.1016/j.tcb.2005.06.006. PMID 16006126.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sarah J. Swansona, Paul C. Bethkea, and Russell L. Jonesa (1998). "Barley Aleurone Cells Contain Two Types of Vacuoles: Characterization of Lytic Organelles by Use of Fluorescent Probes". The Plant Cell. 10 (5): 685–689.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kuehnel, W (2003). Color Atlas of Cytology, Histology, & Microscopic Anatomy (4th ed.). Thieme. p. 34. ISBN 1-58890-175-0.
- ^ a b Alberts, Bruce; et al. (2002). Molecular biology of the cell (4th ed.). New York. ISBN 0-8153-3218-1.
{{cite book}}: Explicit use of et al. in:|first=(help); Unknown parameter|pukblisher=ignored (help)CS1 maint: location missing publisher (link) - ^ a b c d Lodish, Harvey; et al. (2000). Molecular cell biology (4th ed.). New York: Scientific American Books. ISBN 0-7167-3136-3.
{{cite book}}: Explicit use of et al. in:|first=(help) - ^ de Duve C, de Barsy T, Poole B, Trouet A, Tulkens P, van Hoof F. Lysosomotropic agents. Biochem.Pharmacol. 23:2495-2531, 1974. PMID 4606365
- ^ Trapp S, Rosania G, Horobin RW, Kornhuber J. Quantitative modeling of selective lysosomal targeting for drug design. Eur.Biophys.J. 37 (8):1317-1328, 2008. PMID 18504571
- ^ Kornhuber J, Schultz A, Wiltfang J, Meineke I, Gleiter CH, Zöchling R, Boissl KW, Leblhuber F, Riederer P. Persistence of haloperidol in human brain tissue. Am.J.Psychiatry 156:885-890, 1999. PMID 10360127
- ^ Kornhuber J, Weigmann H, Röhrich J, Wiltfang J, Bleich S, Meineke I, Zöchling R, Hartter S, Riederer P, Hiemke C. Region specific distribution of levomepromazine in the human brain. J.Neural Transm. 113:387-397, 2006. PMID 15997416
- ^ Kornhuber J, Quack G, Danysz W, Jellinger K, Danielczyk W, Gsell W, Riederer P. Therapeutic brain concentration of the NMDA receptor antagonist amantadine. Neuropharmacology 34:713-721, 1995. PMID 8532138
- ^ Kornhuber J, Tripal P, Reichel M, Terfloth L, Bleich S, Wiltfang J, Gulbins E. Identification of new functional inhibitors of acid sphingomyelinase using a structure-property-activity relation model. J.Med.Chem. 51:219-237, 2008. PMID 18027916
- ^ Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, Mühle C, Terfloth L, Groemer TW, Spitzer GM, Liedl KR, Gulbins E, Tripal P. Identification of novel functional inhibitors of acid sphingomyelinase. PLoS ONE 6 (8):e23852, 2011. PMID 21909365
- ^ Kornhuber J, Tripal P, Reichel M, Mühle C, Rhein C, Muehlbacher M, Groemer TW, Gulbins E. Functional inhibitors of acid sphingomyelinase (FIASMAs): a novel pharmacological group of drugs with broad clinical applications. Cell.Physiol.Biochem. 26:9-20, 2010. PMID 20502000
External links
 This article incorporates public domain material from Science Primer. NCBI. Archived from the original on 2009-12-08.
This article incorporates public domain material from Science Primer. NCBI. Archived from the original on 2009-12-08.- 3D structures of proteins associated with lysosome membrane
- Hide and Seek Foundation For Lysosomal Research Team
- Self-Destructive Behavior in Cells May Hold Key to a Longer Life
- Mutations in the Lysosomal Enzyme–Targeting Pathway and Persistent Stuttering
- Animation showing how Lysosomes are made, and their function
