Lichen planus
| Lichen planus | |
|---|---|
| Other names | LP |
 | |
| Lichen planus affecting the shins | |
| Pronunciation | |
| Specialty | Dermatology |
Lichen planus (LP) is a chronic inflammatory and autoimmune disease that affects the skin, nails, hair, and mucous membranes.[1][2] It is not an actual lichen, but is named for its appearance.[3] It is characterized by polygonal, flat-topped, violaceous papules and plaques with overlying, reticulated, fine white scale (Wickham's striae), commonly affecting dorsal hands, flexural wrists and forearms, trunk, anterior lower legs and oral mucosa.[4] The hue may be gray-brown in people with darker skin.[5] Although there is a broad clinical range of LP manifestations, the skin and oral cavity remain as the major sites of involvement.[6] The cause is unknown, but it is thought to be the result of an autoimmune process with an unknown initial trigger. There is no cure, but many different medications and procedures have been used in efforts to control the symptoms.
The term lichenoid reaction (lichenoid eruption or lichenoid lesion) refers to a lesion of similar or identical histopathologic and clinical appearance to lichen planus (i.e., an area which resembles lichen planus, both to the naked eye and under a microscope).[7][8] Sometimes dental materials or certain medications can cause lichenoid reactions.[7] They can also occur in association with graft versus host disease.[7][9]: 258
Classification
[edit]Lichen planus lesions are so called because of their "lichen-like" appearance[3] and can be classified by the site they involve, or by their morphology.
Site
[edit]Lichen planus may be categorized as affecting mucosal or cutaneous surfaces.
- Cutaneous forms are those affecting the skin, scalp, and nails.[10][11][12]
- Mucosal forms are those affecting the lining of the gastrointestinal tract (mouth, pharynx, esophagus, stomach, anus), larynx, and other mucosal surfaces including the genitals, peritoneum, ears, nose, bladder and conjunctiva of the eyes.[13][14][15]
Pattern
[edit]Lichen planus lesions can occur in many different forms or morphologies:[16]
- Papular
- Papular form is the classic cutaneous lichen planus (CLP) lesion characterized by shiny, red or purple-colored, flat-topped papule. Lesions may have a thin, transparent, and adherent scale. Fine whitish points or lacy lines (Wickham's striae) may be seen on the surface of well-developed papules.[2]
- Annular
- 'Ring-shaped' lesions that develop gradually from single small pigmented spots into circular groups of papules with clear, unaffected skin in the center. The ring-like lesions may very slowly enlarge, co-join and morph into larger irregular (serpentine) bands, sometimes accompanied by lines (See Linear, below). Annular CLP is uncommon and classically involves the male genitalia (glans penis and penile shaft), groin, axilla and also the extremities.[2]
- Linear
- Papules are arranged in a line (the "Blaschko line").[17] This pattern may develop secondary to trauma (koebnerization) or, uncommonly, as a spontaneous, isolated eruption, usually on the extremities, and rarely on the face.[18]
- Hypertrophic
- This pattern is characterized by hyperkeratotic thick pruritic red-brown to purple-gray plaques with follicular accentuation. Hypertrophic CLP commonly involves the extremities, especially the interphalangeal joints and the anterior legs in a symmetrical distribution.[2] This form is also known as "lichen planus verrucosus".
- Atrophic
- This morphology is characterized by the presence of a few well-demarcated, white-bluish papules or plaques with central superficial atrophy. Atrophic CLP is the clinical endpoint of chronic annular or hypertrophic LP with atrophic lesions. The use of potent topical corticosteroids for a long-term may predispose the patient to developing atrophic lesions.[2]
- Bullous
- This morphology is characterized by the development of vesicles and bullae with the skin lesions. This is a rare variant of lichen planus, and also known as "vesiculobullous lichen planus".
- Actinic
- Rare form presenting as nummular patches or plaques with a hypopigmented halo surrounding a hyperpigmented center. Actinic CLP is more prevalent in African Americans, Indians, and Middle-Eastern individuals and commonly affects the sun-exposed areas.[2]
- Ulcerative
- This morphology is characterized by chronic, painful bullae and ulceration of the feet, often with cicatricial sequelae evident. This is a rare variant of lichen planus.
- Pigmented
- This morphology is characterized by hyperpigmented, dark-brown macules in sun-exposed areas and flexural folds. This is a rare variant of lichen planus.
- Follicular
- Characterized by follicular, flat, elevated or hemispherical erythematous papules with or without keratoses presenting in groups or disseminated. The Graham‐Little‐Piccardi‐Lasseur syndrome, seen in a familial pattern and also predominantly in women, is characterized by the appearance of follicular LP on the trunk with LP follicularis decalvans on the scalp. Follicular LP on the scalp is more likely to lead to scarring alopecia.[16]
- Inverse
- Characterized by extensive erythematous lesions with poorly defined borders and in part with lichenification. Inverse LP typically affects the axillae, inguinal creases, limb flexures and submammary region. Pigmentation of the individual lesions at these inverse locations are typical. Additionally, keratotic papules and erosions with a bizarre configuration can occur.[16]
Overlap syndromes
[edit]Occasionally, lichen planus is known to occur with other conditions. For example:
- Lupus erythematosus overlap syndrome. Lesions of this syndrome share features of both lupus erythematosus and lichen planus. Lesions are usually large and hypopigmented, atrophic, and with a red to blue colour and minimal scaling. Telangectasia may be present.[19][20]
- Lichen sclerosus overlap syndrome, sharing features of lichen planus and lichen sclerosus.[21]
Signs and symptoms
[edit]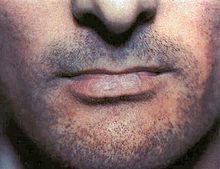
Although lichen planus can present with a variety of lesions, the most common presentation is as a well defined area of purple-coloured, itchy, flat-topped papules with interspersed lacy white lines (Wickham's striae). This description is known as the characteristic "6 Ps" of lichen planus: planar (flat-topped), purple, polygonal, pruritic, papules, and plaques.[10] This rash, after regressing, is likely to leave an area of hyperpigmentation that slowly fades. That said, a variety of other lesions can also occur.[3]
Skin
[edit]

Variants of cutaneous lichen planus are distinguished based upon the appearance of the lesions and/or their distribution.[22] Lesions can affect the:
- Extremities (face, dorsal hands, arms, and nape of neck).[a] This is more common in Middle Eastern countries in spring and summer, where sunlight appears to have a precipitating effect.[18][23][24]
- Palms and soles
- Intertriginous areas of the skin. This is also known as "inverse lichen planus".[18]
- Nails[25] characterized by irregular longitudinal grooving and ridging of the nail plate, thinning of the nail plate, pterygium formation, shedding of the nail plate with atrophy of the nail bed, subungual keratosis, longitudinal erthronychia (red streaks), and subungual hyperpigmentation.[26] A sand-papered appearance is present in around 10% of individuals with nail lichen planus.[25]
- Hair and scalp. The scalp is rarely affected by a condition known as lichen planopilaris, acuminatus, follicular lichen planus, and peripilaris, characterised by violaceous, adherent follicular scale with progressive scarring alopecia. While lichen planus and lichen planopilaris may occur together, aside from sharing the term 'lichen' and revealing inflammation on skin biopsy, there is neither established data on their co-occurrence nor data to suggest a common etiology. Lichen planopilaris is considered an orphan disease with no definitive prevalence data and no proven effective treatments.[27][28]
Other variants may include:
- Lichen planus pemphigoides characterized by the development of tense blisters atop lesions of lichen planus or the development vesicles de novo on uninvolved skin.[29]
- Keratosis lichenoides chronica (also known as "Nekam's disease") is a rare dermatosis characterized by violaceous papular and nodular lesions, often arranged in a linear or reticulate pattern on the dorsal hands and feet, extremities, and buttock, and some cases manifest by seborrheic dermatitis-like eruption on the scalp and face; also palmo plantar keratosis has been reported.[18][30][31]
- Lichenoid keratoses (also known as "benign lichenoid keratosis", and "Solitary lichen planus"[18]) is a cutaneous condition characterized by brown to red scaling maculopapules, found on sun-exposed skin of extremities.[18][32] Restated, this is a cutaneous condition usually characterized by a solitary dusky-red to violaceous papular skin lesion.[33]
- Lichenoid dermatitis represents a wide range of cutaneous disorders characterized by lichen planus-like skin lesions.[18][32]
Mucous membranes
[edit]
Lichen planus affecting mucosal surfaces may have one lesion or be multifocal.[34] Examples of lichen planus affecting mucosal surfaces include:[34]
- Esophageal lichen planus, affecting the esophageal mucosa. This can present with difficulty or pain when swallowing due to oesophageal inflammation, or as the development of an esophageal stricture. It has also been hypothesized that it is a precursor to squamous cell carcinoma of the esophagus.[14][35]
- Genital lichen planus, which may cause lesions on the glans penis or skin of the scrotum in males, and the vulva or vagina in females.[10] Symptoms may include lower urinary tract symptoms associated with stenosis of the urethra, painful sexual intercourse, and itching.[10] In females, Vulvovaginal-gingival syndrome, is severe and distinct variant affecting the vulva, vagina, and gums, with complications including scarring, vaginal stricture formation,[36] or vulva destruction.[37] The corresponding syndrome in males, affecting the glans penis and gums, is the peno-gingival syndrome.[18] It is associated with HLA-DQB1.[18][38]
Mouth
[edit]
Oral lichen planus (also termed oral mucosal lichen planus), is a form of mucosal lichen planus, where lichen planus involves the oral mucosa, the lining of the mouth.[39] This may occur in combination with other variants of lichen planus. Six clinical forms of oral lichen planus (OLP) are recognized:[40]
- Reticular
- The most common presentation of oral lichen planus (OLP) is characterised by the net-like or spider web-like appearance of lacy white lines, known as Wickham's striae.[41] This is usually asymptomatic. Reticular OLP may progress to the more severe subtypes, such as the erosive form, if left untreated.[2]
- Erosive or ulcerative
- The second most common form and the most advanced form of oral lichen planus,[41][2] is characterised by oral ulcers presenting with persistent, irregular areas of redness, ulcerations and erosions covered with a yellow slough. This can occur in one or more areas of the mouth. In 25% of people with erosive oral lichen planus, the gums are involved, described as desquamative gingivitis (a condition not unique to lichen planus). This may be the initial or only sign of the condition.[42] Involvement of the dorsum of the tongue might cause an altered sense of taste (dysgeusia).[2]
- Papular
- This form is characterized by small white pinpoint papules that are asymptomatic. Hence, they can be easily missed during a routine checkup. It is referred to as the initial and transient phase of OLP.[2]
- Plaque-like
- Large, homogenous white patches are characteristic of this form which may resemble leukoplakia. This form is more prevalent in tobacco smokers.[2]
- Atrophic
- This form is a common presentation that has similarities to the erosive form. It has a more prominent atrophic lesion on a background of erythema with radiating white striae at the margins.[2] Atrophic oral lichen planus may also manifest as desquamative gingivitis.[42]
- Bullous
- Rare form of OLP characterized by fluid-filled vesicles ranging in size from 1 to 2 mm to several cm in diameter. The vesicles or bullae appear white or gray-purple in color and are fluctuant. The fluid in the vesicles are usually clear but may be hemorrhagic or purulent upon secondary infection. These rupture easily and they leave an ulcerated, painful surface.[citation needed]
These types often coexist in the same individual. Oral lichen planus (OLP) tends to present bilaterally as mostly white lesions on the inner cheek,[41] although any mucosal site in the mouth may be involved. Other sites, in decreasing order of frequency, may include the tongue, lips, gingivae, floor of the mouth, and very rarely, the palate.[41]
Generally, oral lichen planus tends not to cause any discomfort or pain, although some people may experience soreness when eating or drinking acidic or spicy foodstuffs or beverages.[42] When symptoms arise, they are most commonly associated with the atrophic and ulcerative subtypes. These symptoms can include a burning sensation to severe pain.[41] They may also experience mucosal bleeding in response to mild trauma, such as toothbrushing. Additionally, the Koebner phenomenon (the development of new lesions at sites of trauma) is not only present in cutaneous lichen planus (CLP) but can also occur in the setting of OLP.
Residual postinflammatory hyperpigmentation has been reported in association with OLP, manifesting as brown to black pigmentation on the oral mucosa and may most likely occur in dark-skinned individuals.[43]
OLP may occur as a sole manifestation of the disease or in conjunction with other clinical manifestations of LP, including cutaneous LP, genital LP, nail LP, and lichen planopilaris (scalp LP).[43]
Causes
[edit]Cutaneous LP is a self-limiting condition. It usually resolves within 6 to 12 months. Oral LP is a non infectious, chronic inflammatory condition that involves the oral mucosa and may be accompanied by skin lesions. The etiology of oral LP are unknown.[citation needed]
It is not clear whether the mechanisms causing isolated oral LP are different from those causing oral LP with cutaneous LP. An immune-mediated mechanism where basal keratinocytes are being targeted as foreign antigens by activated T cells, especially CD8+ T cells, has been proposed.[44] Upregulation of intercellular adhesion molecule-1 (ICAM-1) and cytokines associated with T-helper 1 immune response, may also play an important role in the pathogenesis of lichen planus.[citation needed]
Stress is thought to play a role in the pathogenesis of oral LP. Patients with anxiety and depression are reported more commonly with oral LP if compared to normal healthy individuals.[45][46] Some studies have indicated that stressful events can induce LP lesions in otherwise healthy individuals. However, a cause effect relationship between stress and the onset of oral LP has not been demonstrated.[citation needed]
Autoimmune response to epithelial self-antigens remains a possibility. A single study of cutaneous LP reported evidence in support of autoimmunity by expanding in vitro T cells isolated from the skin lesions of two patients, followed by testing the ability of these T cells to kill autologous keratinocytes (cytotoxicity).
Several potential triggers of oral LP have been proposed over the years, mainly
- Hypersensitivity reaction
- Viral infection
Pathogenesis
[edit]Oral LP is considered to be a T-cell mediated chronic inflammatory tissue reaction that results in a cytotoxic reaction against epithelial basal cells.[48] The inflammatory infiltrate in oral LP is primarily composed of CD8+ T cells. A potential pathway for CD8+ T cell-mediated cytotoxicity in oral LP is described as follows:[48]
Antigens presented on MHC 1 molecules activates CD8+ T cells on keratinocytes or by encounters with activated CD4+ helper T cells or cytokines produced by activated CD4+ helper T cells[citation needed]
Activated CD8+ T cells induce keratinocyte apoptosis through various mechanisms such as secretion of tumor necrosis factor (TNF)-alpha, secretion of granzyme B, or Fas-Fas ligand interactions. Chemokines are produced by activated CD8+ T cells that attract additional inflammatory cells, thereby promoting continued inflammation.
Other mechanisms that have been proposed include:
- upregulation of matrix metalloproteinases that disrupt the epithelial basement membrane zone and allow entry of immune cells into the epidermis,
- the release of proinflammatory mediators and proteases by mast cells, and
- perturbations in the innate immune response that may involve toll-like receptors.[48][49][50][51]
Oral LP may also be caused by genetic factor which influence the immune function. A separate study performed in China[52] found an association between a polymorphism in the TNF-alpha gene and risk for oral LP in a subset of patients. An Italian study found a significant increase in a genetic polymorphism of the first intron of the interferon (IFN)-gamma promoter in patients with oral LP compared with controls.[52]
Diagnosis
[edit]Skin
[edit]Patient history and clinical presentation need to be taken to diagnose lichen planus. Patients with suspected cutaneous lichen planus need to be evaluated clinically through patient interviews and physical examinations. Patients should be questioned about their medication history, any history of pruritus or genital pain and history of dysphagia or odynophagia. Examination of entire cutaneous surface including the scalp, oral cavity and external genitalia need to be included. Wickham's striae often can be seen during microscopic examination of cutaneous lesions of lichen planus.[53][54]
To confirm the diagnosis of cutaneous lichen planus, a skin biopsy can be done. A punch biopsy of sufficient depth to the mid dermis is usually significant. Immunofluorescence studies are not always needed. Direct immunofluorescence (DIF) can be useful in patients with bullous lesions to differentiate the condition from an autoimmune vesiculobullous disease.[55]
Mouth
[edit]A diagnosis of oral lichen planus (LP) is confirmed through review of the patient history, physical examination, and histologic findings.[citation needed]
The clinical evaluation should include a patient history that assesses the following:
- History of LP involving other body sites or other skin disorders that may present with similar findings (e.g., autoimmune blistering diseases)
- Presence of associated symptoms (e.g., pain, burning)
- Medication the patients are taking within the few weeks to months after drug initiation e.g. antihypertensives, antidepressants, diuretics, antidiabetics, NSAIDs, etc. to evaluate for the possibility of an oral lichenoid drug eruption
- History of dental restorations,[47] use of dental appliances, or oral exposure to substances that may cause oral lichenoid contact eruptions (e.g. dental composites, cobalt chromium based dentures etc.)
A full examination that includes the evaluation of the mucosal and cutaneous surfaces, including the vulva, vagina, penis, scalp, and nails should be performed. Thorough examination may lead to the detection of extraoral manifestations of LP that provide additional support for the diagnosis or the identification of clinical findings that suggest another diagnosis.
Tissue biopsies of oral LP help to confirm the diagnosis and are particularly of value for erythematous and erosive LP, which share features with multiple other mucosal disorders, including oral malignancy. Biopsies to confirm oral LP are less essential in patients who present with classic reticular LP, particularly in patients in whom a diagnosis of LP has already been confirmed through biopsy of an extraoral manifestation of this disorder.[56][57]
Differential diagnosis
[edit]Skin
[edit]- Lichenoid drug eruption
- The cutaneous manifestations resemble idiopathic lichen planus.
- Chronic graft-versus-host disease
- The history of preceding hematopoietic cell transplant is helpful for diagnosis
- Psoriasis
- Atopic dermatitis
- Cutaneous lupus erythematosus
- Discoid lupus erythematosus[53]
Mouth
[edit]Oral lichenoid drug reaction
[edit]Lichenoid drug eruptions may be caused by a variety of systemic medications and share clinical features with oral LP. Histologic findings of a deep mixed infiltrate with lymphocytes, plasma cells, and neutrophils (with or without eosinophils) and perivascular inflammation favor this diagnosis.
Oral lichenoid contact reaction (allergic contact mucositis)
[edit]Oral lichenoid contact reactions may be caused by a variety of substances. The clinical and histologic features of oral lichenoid contact reactions are similar to oral LP. Patch testing and recognition of the proximity of an offending substance to the eruption can aid with diagnosis.[37]
Autoimmune blistering diseases
[edit]Mucous membrane pemphigoid and other autoimmune blistering diseases may present with oral erosions and desquamative gingivitis similar to that seen in erosive LP. Biopsies for routine histologic examination and direct immunofluorescence are useful for distinguishing these disorders from oral LP.
Graft-versus-host disease (GVHD)
[edit]Lacy, reticulated plaques or erosions that resemble oral LP may occur in GVHD. The histologic findings of these disorders are also similar. The patient history is useful for differentiating chronic GVHD from oral LP.[58] Oral involvement in acute GVHD is less well characterized than chronic GVHD, but has been associated with erythematous, erosive, ulcerative, or lichenoid oral lesions.
Leukoplakia
[edit]
Leukoplakia is a manifestation of squamous epithelial hyperplasia that may be a precursor to oral squamous cell carcinoma. White patches or plaques usually appear on the oral mucosa. To rule out malignancy, a biopsy of leukoplakia is indicated.[59]
Oral squamous cell carcinoma
[edit]Oral squamous cell carcinoma (SCC) can present as erythematous or white patches, ulcers, or exophytic masses. The highest risk for oral SCC may occur in patients with erythematous or erosive oral LP.[60][61][62] A biopsy is indicated.
Leukoedema
[edit]Leukoedema is a common, benign finding in the oral cavity that presents as white-gray, somewhat translucent plaques on the mucosa. The buccal mucosa is the most common site for involvement. Symptoms are absent, and no treatment is necessary.[63][64]
Oropharyngeal candidiasis
[edit]Oropharyngeal candidiasis (also known as thrush) is a common infection that has a predilection for infants, older adults with dentures, immunosuppressed individuals, and individuals utilizing intraoral corticosteroid therapy. Patients present with white plaques or erythematous patches on the buccal mucosa, palate, tongue, or oropharynx that may be mistaken for reticular LP.[65]
Histopathology
[edit]
The histologic findings of oral LP can offer strong support for the diagnosis, but are not pathognomonic. Clinical correlation is required. Common histologic findings of oral LP include:[47]
- Parakeratosis and slight acanthosis of the epithelium
- Saw-toothed rete ridges
- Liquefaction (hydropic) degeneration of the basal layer with apoptotic keratinocytes (referred to as Civatte, colloid, hyaline, or cytoid bodies)
- An amorphous band of eosinophilic material at the basement membrane composed of fibrin or fibrinogen.
- A lichenoid (band-like) lymphocytic infiltrate immediately subjacent to the epithelium.
Nonetheless, interpreting the histopathological features of oral LP has been associated historically with high intra-observer and inter-observer variabilities.[66]
Treatment
[edit]There is no cure for lichen planus,[41] and so treatment of cutaneous and oral lichen planus is for symptomatic relief or due to cosmetic concerns.[3][41][67] When medical treatment is pursued, first-line treatment typically involves either topical or systemic corticosteroids,[3] and removal of any triggers.[68] Without treatment, most lesions will spontaneously resolve within 6–9 months for cutaneous lesions,[3] and longer for mucosal lesions.[69]
Skin
[edit]Many different treatments have been reported for cutaneous lichen planus, however there is a general lack of evidence of efficacy for any treatment.[70][71] The mainstay of localized skin lesions is topical steroids.[41] Additional treatments include retinoids, such as acitretin, or sulfasalazine. Narrow band UVB phototherapy or systemic PUVA therapy are known treatment modalities for generalized disease.[44]
Mouth
[edit]Reassurance that the condition is benign, elimination of precipitating factors and improving oral hygiene are considered initial management for symptomatic OLP, and these measures are reported to be useful.[41] Treatment usually involves topical corticosteroids (such as betamethasone, clobetasol, dexamethasone, and triamcinolone) and analgesics, or if these are ineffective and the condition is severe, then systemic corticosteroids may be used. Calcineurin inhibitors (such as pimecrolimus, tacrolimus or cyclosporin) are sometimes used.[41] While topical steroids are widely accepted as first line treatment for mucosal lichen planus, there is only weak evidence to support their effectiveness for erosive oral lichen planus.[72]
Prognosis
[edit]Cutaneous lichen planus lesions typically resolve within six months to a year. However, some variant such as the hypertrophic variant might persist for years if left untreated or unmonitored.[2]
It is found that cutaneous lichen planus does not carry a risk of skin cancer.[73] In contrast to cutaneous LP, which is self limited, lichen planus lesions in the mouth may persist for many years,[67] and tend to be difficult to treat, with relapses being common.[38][2]
Although this condition was first described almost a century ago, it has been reported that its associated oral cancer risk has been exaggerated.[74] Overall, it is found that patients with erythematous or erosive oral lichen planus have a higher risk of oral squamous cell carcinoma compared to patients diagnosed with other variants.[75]
Due to the possibility that oral LP may increase risk for oral cancer, patients with oral lichen planus are encouraged to avoid activities known to increase the risk for oral cancer, such as smoking and alcohol use.[75][73]
Patients with oral lichen planus should be followed-up at least every 6 to 12 months, to assess the disease activity, changes in symptoms or even detect early signs of malignancy.[75]
Epidemiology
[edit]The overall estimated prevalence of lichen planus in worldwide population is in the range of 0.2% to 5%.[10][76][77][78][79][80]
It generally occurs more commonly in females, in a ratio of 3:2, and most cases are diagnosed between the ages of 30 and 60, but it can occur at any age.[10][81][46]
Lichen planus can occur in patients as diverse cutaneous manifestations alone or in combination with mucosal lichen planus and, or lichen planus of the nails. Study shows that frequency of mucosal involvement of lichen planus patients is 30- 70%.[16]
Oral lichen planus is relatively common,[38] It is one of the most common mucosal diseases. The prevalence in the general population is about 1.27–2.0%,[41][67] and it occurs more commonly in middle aged people.[41] Oral lichen planus in children is rare. About 50% of females with oral lichen planus were reported to have undiagnosed vulvar lichen planus.[10]
Some studies suggest that cutaneous lichen planus is more commonly found in men whilst oral lichen planus lesions are more commonly found in women.[82][83][84][85][86][87]
History
[edit]Lichen planus was first described in 1869 by Erasmus Wilson as an inflammatory disorder with unknown etiology. Initially, the characteristic surface markings or striae was described by Weyl in 1885. In 1895, Wickham further explained the characteristic of the lesion, now known as Wickham striae. Further on, Darier explained the presence of such characteristic markings by correlating with an increase thickness of the granular cell layer. The coexistence of oral, cervical and stomach lichen planus lesions were described by Guogerot and Burnier in 1937. A similar variant of mucosal lichen planus as the vulvovaginal-gingival syndrome with erosive lesions involving oral and vulvovaginal mucosa were introduced by Pelisse and colleagues in year 1982.[2]
The origin of the word is believed to be from the Greek word ''Leichen'', which means tree moss, and also from Latin word ''planus'', which means flat and even surface.
Research
[edit]Apremilast is undergoing investigation as a potential treatment.[88]
Explanatory notes
[edit]- ^ Cutaneous lichen planus affecting the extremities is also known as "lichen planus actinicus", "Actinic lichen niditus", "Lichen planus atrophicus annularis", "Lichen planus subtropicus", "Lichen planus tropicus", "Lichenoid melanodermatitis", and "Summertime actinic lichenoid eruption"
References
[edit]- ^ Wang EH, Monga I, Sallee BN, Chen JC, Abdelaziz AR, Perez-Lorenzo R, Bordone LA, Christiano AM (Jul 2022). "Primary cicatricial alopecias are characterized by dysregulation of shared gene expression pathways". PNAS Nexus. 1 (3): pgac111. doi:10.1093/pnasnexus/pgac111. PMC 9308563. PMID 35899069.
- ^ a b c d e f g h i j k l m n o Gorouhi F, Davari P, Fazel N (2014-01-30). "Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis". The Scientific World Journal. 2014: 742826. doi:10.1155/2014/742826. PMC 3929580. PMID 24672362.
- ^ a b c d e f Therapeutic guidelines (Version 3. ed.). North Melbourne, Vic.: Therapeutic Guidelines. 2009. pp. 254–55, 302. ISBN 978-0-9804764-3-9.
- ^ "Inverse lichen planus: An unusual morphologic variant of a classic papulosquamous dermatosis". Journal of the American Academy of Dermatology. 52 (3): P64. 2005-03-01. doi:10.1016/j.jaad.2004.10.268. ISSN 1097-6787.
- ^ Onalaja, Amanda A.; Taylor, Susan C. (2021). "1. Defining skin color". In Li, Becky S.; Maibach, Howard I. (eds.). Ethnic Skin and Hair and Other Cultural Considerations. Switzerland: Springer. p. 10. ISBN 978-3-030-64829-9.
- ^ Meredith A. Olson; Roy S. Rogers III; Alison J. Bruce (2016). "Oral lichen planus". Clinics in Dermatology.
- ^ a b c Greenberg MS, Glick M, Ship JA (2008). Burket's oral medicine (11th ed.). Hamilton, Ont.: BC Decker. pp. 89–97. ISBN 9781550093452.
- ^ Lewis MA, Jordan RC (2012). Oral medicine (2nd ed.). London: Manson Publishing. pp. 66–72. ISBN 9781840761818.
- ^ Barnes L, ed. (2009). Surgical pathology of the head and neck (3rd ed.). New York: Informa healthcare. ISBN 9781420091632.
- ^ a b c d e f g Le Cleach L, Chosidow O (February 2012). "Clinical practice. Lichen planus". The New England Journal of Medicine. 366 (8): 723–32. doi:10.1056/NEJMcp1103641. PMID 22356325.
- ^ Asch S, Goldenberg G (March 2011). "Systemic treatment of cutaneous lichen planus: an update". Cutis. 87 (3): 129–34. PMID 21488570.
- ^ Sharma A, Białynicki-Birula R, Schwartz RA, Janniger CK (July 2012). "Lichen planus: an update and review". Cutis. 90 (1): 17–23. PMID 22908728.
- ^ Cheng S, Kirtschig G, Cooper S, Thornhill M, Leonardi-Bee J, Murphy R (February 2012). "Interventions for erosive lichen planus affecting mucosal sites" (PDF). The Cochrane Database of Systematic Reviews. 2 (2): CD008092. doi:10.1002/14651858.CD008092.pub2. hdl:1871/48562. PMC 10794897. PMID 22336835.
- ^ a b Yamada T, Alpers DH, et al. (2009). Textbook of gastroenterology (5th ed.). Chichester, West Sussex: Blackwell Pub. p. 3304. ISBN 978-1-4051-6911-0.
- ^ Treister NS, Bruch JM (2010). Clinical oral medicine and pathology. New York: Humana Press. pp. 59–62. ISBN 978-1-60327-519-4.
- ^ a b c d Wagner G, Rose C, Sachse MM (April 2013). "Clinical variants of lichen planus". Journal der Deutschen Dermatologischen Gesellschaft. 11 (4): 309–19. doi:10.1111/ddg.12031. PMID 23320493. S2CID 8927462.
- ^ Arnold, David L.; Krishnamurthy, Karthik (2024), "Lichen Planus", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30252382, retrieved 2024-10-23
- ^ a b c d e f g h i Bolognia, Jean L.; Jorizzo, Joseph L.; Rapini, Ronald P., eds. (2008). Dermatology (2nd ed.). St. Louis: Mosby/Elsevier. ISBN 978-1-4160-2999-1.
- ^ James WD, Elston DM, Berger TG (2011). Andrews' Diseases of the skin : clinical dermatology (11th ed.). London: Saunders/Elsevier. pp. 219–24. ISBN 978-1-4377-0314-6.
- ^ Freedberg IM, ed. (2003). Fitzpatrick's dermatology in general medicine (6th ed.). New York, NY: McGraw-Hill. pp. 366, 470–71. ISBN 978-0-07-138076-8.
- ^ James WD, Elston DM, Berger TG (2011). Andrews' Diseases of the skin : clinical dermatology (11th ed.). London: Saunders/ Elsevier. p. 220. ISBN 978-1-4377-0314-6.
- ^ Freedberg IM, ed. (2003). Fitzpatrick's dermatology in general medicine (6th ed.). New York, NY: McGraw-Hill. p. 466. ISBN 978-0-07-138076-8.
- ^ Freedberg IM, ed. (2003). Fitzpatrick's dermatology in general medicine (6th ed.). New York, NY: McGraw-Hill. p. 468. ISBN 978-0-07-138076-8.
- ^ James, William D.; Elston, Dirk M.; Berger, Timothy G. (2011). Andrews' Diseases of the skin : clinical dermatology (11th ed.). London: Saunders/ Elsevier. p. 223. ISBN 978-1-4377-0314-6.
- ^ a b Gordon KA, Vega JM, Tosti A (Nov–Dec 2011). "Trachyonychia: a comprehensive review". Indian Journal of Dermatology, Venereology and Leprology. 77 (6): 640–5. doi:10.4103/0378-6323.86470. PMID 22016269.
- ^ James WD, Elston DM, Berger TG (2011). Andrews' Diseases of the skin : clinical dermatology (11th ed.). London: Saunders/ Elsevier. p. 781. ISBN 978-1-4377-0314-6.
- ^ "Orphanet: Rare Diseases". Orphanet. Retrieved June 3, 2016.
- ^ "Cicatricial Alopecia Research Foundation". www.carfintl.org. Retrieved June 3, 2016.
- ^ Freedberg IM, ed. (2003). Fitzpatrick's dermatology in general medicine (6th ed.). New York, NY: McGraw-Hill. p. 471. ISBN 978-0-07-138076-8.
- ^ Freedberg IM, ed. (2003). Fitzpatrick's dermatology in general medicine (6th ed.). New York, NY: McGraw-Hill. p. 472. ISBN 978-0-07-138076-8.
- ^ James WD, Elston DM, Berger TG (2011). Andrews' Diseases of the skin : clinical dermatology (11th ed.). London: Saunders/ Elsevier. p. 224. ISBN 978-1-4377-0314-6.
- ^ a b Freedberg IM, ed. (2003). Fitzpatrick's dermatology in general medicine (6th ed.). New York, NY: McGraw-Hill. p. 473. ISBN 978-0-07-138076-8.
- ^ James WD, Elston DM, Berger TG (2011). Andrews' Diseases of the skin : clinical dermatology (11th ed.). London: Saunders/ Elsevier. p. 639. ISBN 978-1-4377-0314-6.
- ^ a b Ebrahimi M, Lundqvist L, Wahlin YB, Nylander E (October 2012). "Mucosal lichen planus, a systemic disease requiring multidisciplinary care: a cross-sectional clinical review from a multidisciplinary perspective". Journal of Lower Genital Tract Disease. 16 (4): 377–80. doi:10.1097/LGT.0b013e318247a907. PMID 22622344. S2CID 25165134.
- ^ Chandan VS, Murray JA, Abraham SC (June 2008). "Esophageal lichen planus". Archives of Pathology & Laboratory Medicine. 132 (6): 1026–9. doi:10.5858/2008-132-1026-ELP. PMID 18517264.
- ^ Panagiotopoulou N, Wong CS, Winter-Roach B (April 2010). "Vulvovaginal-gingival syndrome". Journal of Obstetrics and Gynaecology. 30 (3): 226–30. doi:10.3109/01443610903477572. PMID 20373919. S2CID 45115301.
- ^ a b Schlosser BJ (May–Jun 2010). "Lichen planus and lichenoid reactions of the oral mucosa". Dermatologic Therapy. 23 (3): 251–67. doi:10.1111/j.1529-8019.2010.01322.x. PMID 20597944. S2CID 37730720.
- ^ a b c Nico MM, Fernandes JD, Lourenço SV (Jul–Aug 2011). "Oral lichen planus". Anais Brasileiros de Dermatologia. 86 (4): 633–41, quiz 642–3. doi:10.1590/s0365-05962011000400002. PMID 21987126.
- ^ Alam F, Hamburger J (May 2001). "Oral mucosal lichen planus in children". International Journal of Paediatric Dentistry. 11 (3): 209–14. doi:10.1046/j.1365-263X.2001.00266.x. PMID 11484471.
- ^ Farhi D, Dupin N (2010). "Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies". Clinics in Dermatology. 28 (1): 100–8. doi:10.1016/j.clindermatol.2009.03.004. PMID 20082959.
- ^ a b c d e f g h i j k l Lodi, Giovanni; Manfredi, Maddalena; Mercadante, Valeria; Murphy, Ruth; Carrozzo, Marco (28 February 2020). "Interventions for treating oral lichen planus: corticosteroid therapies". The Cochrane Database of Systematic Reviews. 2020 (2): CD001168. doi:10.1002/14651858.CD001168.pub3. ISSN 1469-493X. PMC 7047223. PMID 32108333.
- ^ a b c Scully C (2008). Oral and maxillofacial medicine : the basis of diagnosis and treatment (3rd ed.). Edinburgh: Churchill Livingstone. pp. 192–99. ISBN 9780702049484.
- ^ a b "UpToDate". www.uptodate.com. Retrieved 2019-01-04.
- ^ a b Lehman JS, Tollefson MM, Gibson LE (July 2009). "Lichen planus". International Journal of Dermatology. 48 (7): 682–94. doi:10.1111/j.1365-4632.2009.04062.x. PMID 19570072. S2CID 35881020.
- ^ McCartan BE (July 1995). "Psychological factors associated with oral lichen planus". Journal of Oral Pathology & Medicine. 24 (6): 273–5. doi:10.1111/j.1600-0714.1995.tb01181.x. PMID 7562664.
- ^ a b Eisen D (February 2002). "The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients". Journal of the American Academy of Dermatology. 46 (2): 207–14. doi:10.1067/mjd.2002.120452. PMID 11807431.
- ^ a b c Schlosser BJ (May 2010). "Lichen planus and lichenoid reactions of the oral mucosa". Dermatologic Therapy. 23 (3): 251–67. doi:10.1111/j.1529-8019.2010.01322.x. PMID 20597944. S2CID 37730720.
- ^ a b c Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A (November 2010). "Pathogenesis of oral lichen planus--a review". Journal of Oral Pathology & Medicine. 39 (10): 729–34. doi:10.1111/j.1600-0714.2010.00946.x. PMID 20923445. S2CID 3384200.
- ^ Siponen M, Kauppila JH, Soini Y, Salo T (November 2012). "TLR4 and TLR9 are induced in oral lichen planus". Journal of Oral Pathology & Medicine. 41 (10): 741–7. doi:10.1111/j.1600-0714.2012.01169.x. PMID 22672741.
- ^ El Tawdy A, Rashed L (July 2012). "Downregulation of TLR-7 receptor in hepatic and non-hepatic patients with lichen planus". International Journal of Dermatology. 51 (7): 785–9. doi:10.1111/j.1365-4632.2011.04977.x. PMID 22715821. S2CID 34765371.
- ^ Janardhanam SB, Prakasam S, Swaminathan VT, Kodumudi KN, Zunt SL, Srinivasan M (May 2012). "Differential expression of TLR-2 and TLR-4 in the epithelial cells in oral lichen planus". Archives of Oral Biology. 57 (5): 495–502. doi:10.1016/j.archoralbio.2011.10.013. PMID 22119043.
- ^ a b Carrozzo M, Uboldi de Capei M, Dametto E, Fasano ME, Arduino P, Broccoletti R, Vezza D, Rendine S, Curtoni ES, Gandolfo S (January 2004). "Tumor necrosis factor-alpha and interferon-gamma polymorphisms contribute to susceptibility to oral lichen planus". The Journal of Investigative Dermatology. 122 (1): 87–94. doi:10.1046/j.0022-202X.2003.22108.x. PMID 14962095.
- ^ a b Lallas, A.; Kyrgidis, A.; Tzellos, T. G.; Apalla, Z.; Karakyriou, E.; Karatolias, A.; Lefaki, I.; Sotiriou, E.; Ioannides, D. (June 2012). "Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea". The British Journal of Dermatology. 166 (6): 1198–1205. doi:10.1111/j.1365-2133.2012.10868.x. ISSN 1365-2133. PMID 22296226. S2CID 1468632.
- ^ Friedman, Paula; Sabban, Emilia Cohen; Marcucci, Carolina; Peralta, Rosario; Cabo, Horacio (October 2015). "Dermoscopic findings in different clinical variants of lichen planus. Is dermoscopy useful?". Dermatology Practical & Conceptual. 5 (4): 51–55. doi:10.5826/dpc.0504a13. ISSN 2160-9381. PMC 4667604. PMID 26693092.
- ^ Weedon, David (2010), "The lichenoid reaction pattern ('interface dermatitis')", Weedon's Skin Pathology, Elsevier, pp. 35–70.e41, doi:10.1016/b978-0-7020-3485-5.00004-8, ISBN 9780702034855
- ^ Agha R, Mirowski GW (May 2010). "The art and science of oral examination". Dermatologic Therapy. 23 (3): 209–19. doi:10.1111/j.1529-8019.2010.01318.x. PMID 20597940. S2CID 6367644.
- ^ Eisen D (June 1992). "The oral mucosal punch biopsy. A report of 140 cases". Archives of Dermatology. 128 (6): 815–7. doi:10.1001/archderm.1992.01680160099013. PMID 1599270.
- ^ Vargas-Díez E, García-Díez A, Marín A, Fernández-Herrera J (May 2005). "Life-threatening graft-vs-host disease". Clinics in Dermatology. 23 (3): 285–300. doi:10.1016/j.clindermatol.2004.06.005. PMID 15896544.
- ^ Warnakulasuriya S, Johnson NW, van der Waal I (November 2007). "Nomenclature and classification of potentially malignant disorders of the oral mucosa". Journal of Oral Pathology & Medicine. 36 (10): 575–80. doi:10.1111/j.1600-0714.2007.00582.x. PMID 17944749.
- ^ van der Meij EH, Mast H, van der Waal I (September 2007). "The possible premalignant character of oral lichen planus and oral lichenoid lesions: a prospective five-year follow-up study of 192 patients". Oral Oncology. 43 (8): 742–8. doi:10.1016/j.oraloncology.2006.09.006. PMID 17112770.
- ^ Hietanen J, Paasonen MR, Kuhlefelt M, Malmström M (May 1999). "A retrospective study of oral lichen planus patients with concurrent or subsequent development of malignancy". Oral Oncology. 35 (3): 278–82. doi:10.1016/S1368-8375(98)00116-X. PMID 10621848.
- ^ Parashar P (February 2011). "Oral lichen planus". Otolaryngologic Clinics of North America. 44 (1): 89–107, vi. doi:10.1016/j.otc.2010.09.004. PMID 21093625.
- ^ Duncan SC, Su WP (August 1980). "Leukoedema of the oral mucosa. Possibly an acquired white sponge nevus". Archives of Dermatology. 116 (8): 906–8. doi:10.1001/archderm.1980.01640320056014. PMID 7406518.
- ^ Martin JL (November 1992). "Leukoedema: a review of the literature". Journal of the National Medical Association. 84 (11): 938–40. PMC 2571748. PMID 1460680.
- ^ Shay K, Truhlar MR, Renner RP (July 1997). "Oropharyngeal candidosis in the older patient" (PDF). Journal of the American Geriatrics Society. 45 (7): 863–70. doi:10.1111/j.1532-5415.1997.tb01517.x. hdl:2027.42/111172. PMID 9215341. S2CID 46075976.
- ^ Majdy Idrees, Camile S Farah, Syed Ali Khurram, Norman Firth, Merva Soluk-Tekkesin, Omar Kujan. 2021, Observer agreement in the diagnosis of oral lichen planus using the proposed criteria of the American Academy of Oral and Maxillofacial Pathology. J Oral Pathol Med 2021 Mar 17. doi:10.1111/jop.13170
- ^ a b c Kerawala C, Newlands C, eds. (2010). Oral and maxillofacial surgery. Oxford: Oxford University Press. pp. 412–13. ISBN 978-0-19-920483-0.
- ^ Issa Y, Brunton PA, Glenny AM, Duxbury AJ (November 2004). "Healing of oral lichenoid lesions after replacing amalgam restorations: a systematic review". Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 98 (5): 553–65. doi:10.1016/j.tripleo.2003.12.027. PMID 15529127.
- ^ Scully, C.; El-Kom, M. (1 July 1985). "Lichen planus: review and update on pathogenesis". Journal of Oral Pathology and Medicine. 14 (6): 431–58. doi:10.1111/j.1600-0714.1985.tb00516.x. PMID 3926971.
- ^ Cribier B, Frances C, Chosidow O (December 1998). "Treatment of lichen planus. An evidence-based medicine analysis of efficacy". Archives of Dermatology. 134 (12): 1521–30. doi:10.1001/archderm.134.12.1521. PMID 9875189.
- ^ Antiga E, Caproni M, Parodi A, Cianchini G, Fabbri P (December 2014). "Treatment of cutaneous lichen planus: an evidence based analysis of efficacy by the Italian Group for Cutaneous Immunopathology". Giornale Italiano di Dermatologia e Venereologia. 149 (6): 719–26. PMID 25664824.
- ^ Cheng S, Kirtschig G, Cooper S, Thornhill M, Leonardi-Bee J, Murphy R (February 2012). "Interventions for erosive lichen planus affecting mucosal sites". The Cochrane Database of Systematic Reviews. 2015 (2): CD008092. doi:10.1002/14651858.CD008092.pub2. hdl:1871/48562. PMC 10794897. PMID 22336835.
- ^ a b Tsu-Yi Chuang (Feb 24, 2020). "Lichen Planus: Practice Essentials, Background, Pathophysiology".
- ^ Idrees, M; Kujan, O; Shearston, K; Farah, CS (25 January 2020). "Oral lichen planus has a very low malignant transformation rate: A systematic review and meta-analysis using strict diagnostic and inclusion criteria". Journal of Oral Pathology & Medicine. 50 (3): 287–298. doi:10.1111/jop.12996. PMID 31981238. S2CID 210891674.
- ^ a b c "UpToDate". www.uptodate.com. Retrieved 2019-01-07.
- ^ Miller CS, Epstein JB, Hall EH, Sirois D (January 2001). "Changing oral care needs in the United States: the continuing need for oral medicine". Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 91 (1): 34–44. doi:10.1067/moe.2001.110439. PMID 11174569.
- ^ Bouquot JE, Gorlin RJ (April 1986). "Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years". Oral Surgery, Oral Medicine, and Oral Pathology. 61 (4): 373–81. doi:10.1016/0030-4220(86)90422-6. PMID 3458148.
- ^ Axéll T, Rundquist L (February 1987). "Oral lichen planus--a demographic study". Community Dentistry and Oral Epidemiology. 15 (1): 52–6. doi:10.1111/j.1600-0528.1987.tb00480.x. PMID 3467894.
- ^ Alabi GO, Akinsanya JB (June 1981). "Lichen planus in tropical Africa". Tropical and Geographical Medicine. 33 (2): 143–7. PMID 7281214.
- ^ Li TJ, Cui J (August 2013). "COX-2, MMP-7 expression in oral lichen planus and oral squamous cell carcinoma". Asian Pacific Journal of Tropical Medicine. 6 (8): 640–3. doi:10.1016/s1995-7645(13)60110-8. PMID 23790336.
- ^ Yu TC, Kelly SC, Weinberg JM, Scheinfeld NS (March 2003). "Isolated lichen planus of the lower lip". Cutis. 71 (3): 210–2. PMID 12661749.
- ^ McCartan BE, Healy CM (September 2008). "The reported prevalence of oral lichen planus: a review and critique". Journal of Oral Pathology & Medicine. 37 (8): 447–53. doi:10.1111/j.1600-0714.2008.00662.x. PMID 18624932.
- ^ Carbone M, Arduino PG, Carrozzo M, Gandolfo S, Argiolas MR, Bertolusso G, Conrotto D, Pentenero M, Broccoletti R (April 2009). "Course of oral lichen planus: a retrospective study of 808 northern Italian patients". Oral Diseases. 15 (3): 235–43. doi:10.1111/j.1601-0825.2009.01516.x. PMID 19222766.
- ^ Hellgren L (December 1970). "The prevalence of rheumatoid arthritis in different geographical areas in Sweden". Acta Rheumatologica Scandinavica. 16 (4): 293–303. doi:10.3109/rhe1.1970.16.issue-1-4.34. PMID 5493043.
- ^ Nagao T, Ikeda N, Fukano H, Hashimoto S, Shimozato K, Warnakulasuriya S (October 2005). "Incidence rates for oral leukoplakia and lichen planus in a Japanese population". Journal of Oral Pathology & Medicine. 34 (9): 532–9. doi:10.1111/j.1600-0714.2005.00349.x. PMID 16138891.
- ^ Xue JL, Fan MW, Wang SZ, Chen XM, Li Y, Wang L (September 2005). "A clinical study of 674 patients with oral lichen planus in China". Journal of Oral Pathology & Medicine. 34 (8): 467–72. doi:10.1111/j.1600-0714.2005.00341.x. PMID 16091113.
- ^ Thorn JJ, Holmstrup P, Rindum J, Pindborg JJ (May 1988). "Course of various clinical forms of oral lichen planus. A prospective follow-up study of 611 patients". Journal of Oral Pathology. 17 (5): 213–8. doi:10.1111/j.1600-0714.1988.tb01527.x. PMID 3144584.
- ^ Paul J, Foss CE, Hirano SA, Cunningham TD, Pariser DM (February 2013). "An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series". Journal of the American Academy of Dermatology. 68 (2): 255–61. doi:10.1016/j.jaad.2012.07.014. PMID 22910104.
External links
[edit] Media related to Lichen planus at Wikimedia Commons
Media related to Lichen planus at Wikimedia Commons
