Lithium peroxide

| |
 | |
| Names | |
|---|---|
| IUPAC name
Lithium peroxide
| |
| Other names
Dilithium peroxide
Lithium(I) peroxide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.585 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Li2O2 | |
| Molar mass | 45.885 g/mol |
| Appearance | fine, white powder |
| Odor | odorless |
| Density | 2.32 g/cm3[1][2] |
| Melting point | Decomposes to Li2O at ~450°C but melts at 197°C[3] |
| Boiling point | NA |
| soluble[vague] | |
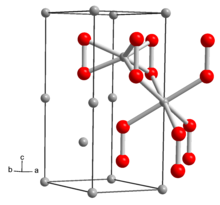
| Structure | |
| hexagonal | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-13.83 kJ/g |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H271, H272, H314 | |
| P210, P220, P221, P260, P264, P280, P283, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P306+P360, P310, P321, P363, P370+P378, P371+P380+P375, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other cations
|
Sodium peroxide Potassium peroxide Rubidium peroxide Caesium peroxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lithium peroxide is the inorganic compound with the formula Li2O2. Lithium peroxide is a white solid, and unlike most other alkali metal peroxides, it is nonhygroscopic. Because of its high oxygen:mass and oxygen:volume ratios, the solid has been used to remove CO2 from and release O2 to the atmosphere in spacecraft.[4]
Preparation
[edit]It is prepared by the reaction of hydrogen peroxide and lithium hydroxide. This reaction initially produces lithium hydroperoxide:[4][5]
- LiOH + H2O2 → LiOOH + H2O
This lithium hydroperoxide may exist as lithium peroxide monoperoxohydrate trihydrate (Li2O2·H2O2·3H2O). Dehydration of this material gives the anhydrous peroxide salt:
- 2 LiOOH → Li2O2 + H2O2
Li2O2 decomposes at about 450 °C to give lithium oxide:
- 2 Li2O2 → 2 Li2O + O2
The structure of solid Li2O2 has been determined by X-ray crystallography and density functional theory. The solid features eclipsed "ethane-like" Li6O2 subunits with an O-O distance of around 1.5 Å.[6]
Uses
[edit]Air Purification
[edit]It is used in air purifiers where weight is important, e.g., spacecraft or other sealed spaces and apparatuses to absorb carbon dioxide and release oxygen in the reaction:[4]
Li2O2 + CO2 → Li2CO3 + 1⁄2 O2
Similar to the reaction of lithium hydroxide with carbon dioxide to release 1 Li2CO3 and 1 H2O, lithium peroxide has high absorption capacity and absorbs more CO2 than does the same weight of lithium hydroxide and offers the bonus of releasing oxygen instead of water. [7]
Polymerization of Styrene
[edit]Lithium peroxide can also act as a catalyst for polymerization of styrene to polystyrene. The polymerization of styrene to polystyrene typically involves the use of radical initiators via the free radical chain mechanism but lithium peroxide can also initiate radical polymerization reactions under certain conditions, although not as widely used.
Lithium-air Battery
[edit]The reversible lithium peroxide reaction is the basis for a prototype lithium–air battery. Using oxygen from the atmosphere allows the battery to eliminate storage of oxygen for its reaction, saving battery weight and size.[8]
See also
[edit]References
[edit]- ^ "Physical Constants of Inorganic Compounds," in CRC Handbook of Chemistry and Physics, 91st Edition (Internet Version 2011), W. M. Haynes, ed., CRC Press/Taylor and Francis, Boca Raton, Florida. (pp: 4-72).
- ^ Speight, James G. (2005). Lange's Handbook of Chemistry (16th Edition). (pp: 1.40). McGraw-Hill. Online version available at: http://www.knovel.com/web/portal/browse/display?_EXT_KNOVEL_DISPLAY_bookid=1347&VerticalID=0
- ^ Phys.Chem.Chem.Phys.,2013,15, 11025. doi:10.1039/c3cp51056e
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 98. ISBN 978-0-08-022057-4.
- ^ E. Dönges "Lithium and Sodium Peroxides" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 979.
- ^ L. G. Cota and P. de la Mora "On the structure of lithium peroxide, Li2O2" Acta Crystallogr. 2005, vol. B61, pages 133-136. doi:10.1107/S0108768105003629
- ^ Ulrich Wietelmann, Richard J. Bauer "Lithium and Lithium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH: Weinheim. doi:10.1002/14356007.a15_393.pub2
- ^ Girishkumar, G.; B. McCloskey; AC Luntz; S. Swanson; W. Wilcke (July 2, 2010). "Lithium- air battery: Promise and challenges". The Journal of Physical Chemistry Letters. 1 (14): 2193–2203. doi:10.1021/jz1005384.

