Cysteine
 Skeletal formula of L-cysteine
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Cysteine
| |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | Cys, C | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.145 | ||
| EC Number |
| ||
| E number | E920 (glazing agents, ...) | ||
| KEGG | |||
PubChem CID
|
|||
| UNII |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties[4] | |||
| C3H7NO2S | |||
| Molar mass | 121.15 g·mol−1 | ||
| Appearance | white crystals or powder | ||
| Melting point | 240 °C (464 °F; 513 K) decomposes | ||
| 277g/L (at 25 °C)[1] | |||
| Solubility | 1.5g/100g ethanol 19 °C [2] | ||
| Acidity (pKa) | 1.71 (conjugate acid), 8.33 (thiol), 10.78[3] | ||
Chiral rotation ([α]D)
|
+9.4° (H2O, c = 1.3) | ||
| Supplementary data page | |||
| Cysteine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Cysteine (symbol Cys or C;[5] /ˈsɪstɪiːn/)[6] is a semiessential[7] proteinogenic amino acid with the formula HOOC−CH(−NH2)−CH2−SH. The thiol side chain in cysteine enables the formation of disulfide bonds, and often participates in enzymatic reactions as a nucleophile. Cysteine is chiral, but both D and L-cysteine are found in nature. L‑Cysteine is a protein monomer in all biota, and D-cysteine acts as a signaling molecule in mammalian nervous systems.[8] Cysteine is named after its discovery in urine, which comes from the urinary bladder or cyst, from Greek κύστη kýsti, "bladder".[9]
The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used.[10][11] The deprotonated form can generally be described by the symbol Cym as well.[11][12]
When used as a food additive, cysteine has the E number E920.
Cysteine is encoded by the codons UGU and UGC.
Structure
[edit]Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has l chirality in the older d/l notation based on homology to d- and l-glyceraldehyde. In the newer R/S system of designating chirality, based on the atomic numbers of atoms near the asymmetric carbon, cysteine (and selenocysteine) have R chirality, because of the presence of sulfur (or selenium) as a second neighbor to the asymmetric carbon atom. The remaining chiral amino acids, having lighter atoms in that position, have S chirality. Replacing sulfur with selenium gives selenocysteine.
Dietary sources
[edit]Cysteinyl is a residue in high-protein foods. Some foods considered rich in cysteine include poultry, eggs, beef, and whole grains. In high-protein diets, cysteine may be partially responsible for reduced blood pressure and stroke risk.[13] Although classified as a nonessential amino acid,[14] in rare cases, cysteine may be essential for infants, the elderly, and individuals with certain metabolic diseases or who suffer from malabsorption syndromes. Cysteine can usually be synthesized by the human body under normal physiological conditions if a sufficient quantity of methionine is available.
Industrial sources
[edit]The majority of l-cysteine is obtained industrially by hydrolysis of animal materials, such as poultry feathers or hog hair. Despite widespread rumor,[15] human hair is rarely a source material.[16] Indeed, food additive or cosmetic product manufactures may not legally source from human hair in the European Union.[17][18]
Some animal-originating sources of l-cysteine as a food additive contravene kosher, halal, vegan, or vegetarian diets.[15] To avoid this problem, synthetic l-cysteine, compliant with Jewish kosher and Muslim halal laws, is also available, albeit at a higher price.[19] The typical synthetic route involves fermentation with an artificial E. coli strain.[20]
Alternatively, Evonik (formerly Degussa) introduced a route from substituted thiazolines.[21] Pseudomonas thiazolinophilum hydrolyzes racemic 2‑amino-Δ2‑thiazoline-4‑carboxylic acid to l‑cysteine.[20]
Biosynthesis
[edit]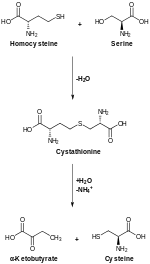
In animals, biosynthesis begins with the amino acid serine. The sulfur is derived from methionine, which is converted to homocysteine through the intermediate S-adenosylmethionine. Cystathionine beta-synthase then combines homocysteine and serine to form the asymmetrical thioether cystathionine. The enzyme cystathionine gamma-lyase converts the cystathionine into cysteine and alpha-ketobutyrate. In plants and bacteria, cysteine biosynthesis also starts from serine, which is converted to O-acetylserine by the enzyme serine transacetylase. The enzyme cysteine synthase, using sulfide sources, converts this ester into cysteine, releasing acetate.[22]
Biological functions
[edit]The cysteine sulfhydryl group is nucleophilic and easily oxidized. The reactivity is enhanced when the thiol is ionized, and cysteine residues in proteins have pKa values close to neutrality, so are often in their reactive thiolate form in the cell.[23] Because of its high reactivity, the sulfhydryl group of cysteine has numerous biological functions.
Precursor to the antioxidant glutathione
[edit]Due to the ability of thiols to undergo redox reactions, cysteine and cysteinyl residues have antioxidant properties. Its antioxidant properties are typically expressed in the tripeptide glutathione, which occurs in humans and other organisms. The systemic availability of oral glutathione (GSH) is negligible; so it must be biosynthesized from its constituent amino acids, cysteine, glycine, and glutamic acid. While glutamic acid is usually sufficient because amino acid nitrogen is recycled through glutamate as an intermediary, dietary cysteine and glycine supplementation can improve synthesis of glutathione.[24]
Precursor to iron-sulfur clusters
[edit]Cysteine is an important source of sulfide in human metabolism. The sulfide in iron-sulfur clusters and in nitrogenase is extracted from cysteine, which is converted to alanine in the process.[25]
Metal ion binding
[edit]Beyond the iron-sulfur proteins, many other metal cofactors in enzymes are bound to the thiolate substituent of cysteinyl residues. Examples include zinc in zinc fingers and alcohol dehydrogenase, copper in the blue copper proteins, iron in cytochrome P450, and nickel in the [NiFe]-hydrogenases.[26] The sulfhydryl group also has a high affinity for heavy metals, so that proteins containing cysteine, such as metallothionein, will bind metals such as mercury, lead, and cadmium tightly.[27]
Roles in protein structure
[edit]In the translation of messenger RNA molecules to produce polypeptides, cysteine is coded for by the UGU and UGC codons.
Cysteine has traditionally been considered to be a hydrophilic amino acid, based largely on the chemical parallel between its sulfhydryl group and the hydroxyl groups in the side chains of other polar amino acids. However, the cysteine side chain has been shown to stabilize hydrophobic interactions in micelles to a greater degree than the side chain in the nonpolar amino acid glycine and the polar amino acid serine.[28] In a statistical analysis of the frequency with which amino acids appear in various proteins, cysteine residues were found to associate with hydrophobic regions of proteins. Their hydrophobic tendency was equivalent to that of known nonpolar amino acids such as methionine and tyrosine (tyrosine is polar aromatic but also hydrophobic[29]), those of which were much greater than that of known polar amino acids such as serine and threonine.[30] Hydrophobicity scales, which rank amino acids from most hydrophobic to most hydrophilic, consistently place cysteine towards the hydrophobic end of the spectrum, even when they are based on methods that are not influenced by the tendency of cysteines to form disulfide bonds in proteins. Therefore, cysteine is now often grouped among the hydrophobic amino acids,[31][32] though it is sometimes also classified as slightly polar,[33] or polar.[7]
Most cysteine residues are covalently bonded to other cysteine residues to form disulfide bonds, which play an important role in the folding and stability of some proteins, usually proteins secreted to the extracellular medium.[34] Since most cellular compartments are reducing environments, disulfide bonds are generally unstable in the cytosol with some exceptions as noted below.

Disulfide bonds in proteins are formed by oxidation of the sulfhydryl group of cysteine residues. The other sulfur-containing amino acid, methionine, cannot form disulfide bonds. More aggressive oxidants convert cysteine to the corresponding sulfinic acid and sulfonic acid. Cysteine residues play a valuable role by crosslinking proteins, which increases the rigidity of proteins and also functions to confer proteolytic resistance (since protein export is a costly process, minimizing its necessity is advantageous). Inside the cell, disulfide bridges between cysteine residues within a polypeptide support the protein's tertiary structure. Insulin is an example of a protein with cystine crosslinking, wherein two separate peptide chains are connected by a pair of disulfide bonds.
Protein disulfide isomerases catalyze the proper formation of disulfide bonds; the cell transfers dehydroascorbic acid to the endoplasmic reticulum, which oxidizes the environment. In this environment, cysteines are, in general, oxidized to cystine and are no longer functional as a nucleophiles.
Aside from its oxidation to cystine, cysteine participates in numerous post-translational modifications. The nucleophilic sulfhydryl group allows cysteine to conjugate to other groups, e.g., in prenylation. Ubiquitin ligases transfer ubiquitin to its pendant, proteins, and caspases, which engage in proteolysis in the apoptotic cycle. Inteins often function with the help of a catalytic cysteine. These roles are typically limited to the intracellular milieu, where the environment is reducing, and cysteine is not oxidized to cystine.
Evolutionary role of cysteine
[edit]Cysteine is considered a "newcomer" amino acid, being the 17th amino acid incorporated into the genetic code.[35][36] Similar to other later-added amino acids such as methionine, tyrosine, and tryptophan, cysteine exhibits strong nucleophilic and redox-active properties.[37][38] These properties contribute to the depletion of cysteine from respiratory chain complexes, such as Complexes I and IV,[39] since reactive oxygen species (ROS) produced by the respiratory chain can react with the cysteine residues in these complexes, leading to dysfunctional proteins and potentially contributing to aging. The primary response of a protein to ROS is the oxidation of cysteine and the loss of free thiol groups,[40] resulting in increased thiyl radicals and associated protein cross-linking.[41][42] In contrast, another sulfur-containing, redox-active amino acid, methionine, does not exhibit these biochemical properties and its content is relatively upregulated in mitochondrially encoded proteins.[43]
Applications
[edit]Cysteine, mainly the l-enantiomer, is a precursor in the food, pharmaceutical, and personal-care industries. One of the largest applications is the production of flavors. For example, the reaction of cysteine with sugars in a Maillard reaction yields meat flavors.[44] l-Cysteine is also used as a processing aid for baking.[45]
In the field of personal care, cysteine is used for permanent-wave applications, predominantly in Asia. Again, the cysteine is used for breaking up the disulfide bonds in the hair's keratin.
Cysteine is a very popular target for site-directed labeling experiments to investigate biomolecular structure and dynamics. Maleimides selectively attach to cysteine using a covalent Michael addition. Site-directed spin labeling for EPR or paramagnetic relaxation-enhanced NMR also uses cysteine extensively.
Reducing toxic effects of alcohol
[edit]Cysteine has been proposed as a preventive or antidote for some of the negative effects of alcohol, including liver damage and hangover. It counteracts the poisonous effects of acetaldehyde.[46] It binds to acetaldehyde to form the low-toxicity heterocycle methylthioproline.[47]
In a rat study, test animals received an LD90 dose of acetaldehyde. Those that received cysteine had an 80% survival rate; when both cysteine and thiamine were administered, all animals survived. The control group had a 10% survival rate.[48]
In 2020 an article was published that suggests L-cysteine might also work in humans.[49]
N-Acetylcysteine
[edit]N-Acetyl-l-cysteine is a derivative of cysteine wherein an acetyl group is attached to the nitrogen atom. This compound is sold as a dietary supplement, and used as an antidote in cases of acetaminophen overdose.[50]
Sheep
[edit]Cysteine is required by sheep to produce wool. It is an essential amino acid that is taken in from their feed. As a consequence, during drought conditions, sheep produce less wool; however, transgenic sheep that can make their own cysteine have been developed.[51]
Chemical reactions
[edit]Being multifunctional, cysteine undergoes a variety of reactions. Much attention has focused on protecting the sulfhydryl group.[52] Methylation of cysteine gives S-methylcysteine. Treatment with formaldehyde gives the thiazolidine thioproline. Cysteine forms a variety of coordination complexes upon treatment with metal ions.[53]
Safety
[edit]Relative to most other amino acids, cysteine is much more toxic.[54]
History
[edit]This section needs expansion. You can help by adding to it. (January 2023) |
In 1884 German chemist Eugen Baumann found that when cystine was treated with a reducing agent, cystine revealed itself to be a dimer of a monomer which he named "cysteïne".[55]
See also
[edit]References
[edit]- ^ "PubChem data".
- ^ Belitz, H.-D; Grosch, Werner; Schieberle, Peter (2009-02-27). Food Chemistry. Springer. ISBN 9783540699330.
- ^ Kirste, Burkhard (23 Jan 1998). "Cysteine". Overview of Amino Acids. Free University of Berlin Dep't. of Biology, Chemistry, and Pharmacy. Archived 2016-11-10 at the Wayback Machine
- ^ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, Florida: CRC Press. p. C-259. ISBN 0-8493-0462-8..
- ^ "Nomenclature and symbolism for amino acids and peptides (IUPAC-IUB Recommendations 1983)". Pure and Applied Chemistry. 56 (5): 595–624. 1984. doi:10.1351/pac198456050595.
- ^ "cysteine - Definition of cysteine in English by Oxford Dictionaries". Oxford Dictionaries - English. Archived from the original on September 25, 2016. Retrieved 15 April 2018.
- ^ a b "The primary structure of proteins is the amino acid sequence". The Microbial World. University of Wisconsin-Madison Bacteriology Department. Archived from the original on 25 May 2013. Retrieved 16 September 2012.
- ^ Semenza, Evan R.; Harraz, Maged M.; Abramson, Efrat; Malla, Adarsha P.; Vasavda, Chirag; Gadalla, Moataz M.; Kornberg, Michael D.; Snyder, Solomon H.; Roychaudhuri, Robin (23 Sep 2021) [18 Aug 2021]. "D-cysteine is an endogenous regulator of neural progenitor cell dynamics in the mammalian brain". PNAS. 118 (39): e2110610118. Bibcode:2021PNAS..11810610S. doi:10.1073/pnas.2110610118. PMC 8488618. PMID 34556581.
- ^ Saffran, M. (April 1998). "Amino acid names and parlor games: from trivial names to a one-letter code, amino acid names have strained students' memories. Is a more rational nomenclature possible?". Biochemical Education. 26 (2): 116–118. doi:10.1016/s0307-4412(97)00167-2. ISSN 0307-4412.
- ^ "Amber Workshop - Tutorial A1 - Section 1: Do some editing of the PDB file". ambermd.org. Archived from the original on 2022-05-22. Retrieved 2022-06-02.
- ^ a b Lee, Jumin; Hitzenberger, Manuel; Rieger, Manuel; Kern, Nathan R.; Zacharias, Martin; Im, Wonpil (21 July 2020). "CHARMM-GUI supports the Amber force fields". The Journal of Chemical Physics. 153 (3): 035103. doi:10.1063/5.0012280. PMID 32716185. S2CID 220796795.
- ^ "Amber Workshop - Tutorial A1 - Section 1: Do some editing of the PDB file". ambermd.org. Archived from the original on 2022-05-22. Retrieved 2022-06-02.
- ^ Larsson, Susanna C.; Håkansson, Niclas; Wolk, Alicja (April 2015). "Dietary Cysteine and Other Amino Acids and Stroke Incidence in Women". Stroke. 46 (4): 922–926. doi:10.1161/STROKEAHA.114.008022. PMID 25669310. S2CID 14895681.
- ^ "Cysteine - Health Encyclopedia - University of Rochester Medical Center". www.urmc.rochester.edu. Retrieved 2024-05-13.
- ^ a b See, e.g., Blech, Zushe Yosef (May 2003). "Like mountains hanging by a hair: Kosher issues in L-cysteine (Commentary on Chagiga I:8)". MK News and Views. Vol. IV, no. 6. Montreal Kosher – via kashrut.com. Rabbi Blech does not address hog hair-derived cysteine, which is almost certainly treyf.
- ^ "VRG-News July 2007 -- the Vegetarian Resource Group". vrg.org. Retrieved 26 August 2024.
- ^ "EU Chemical Requirements". Retrieved May 24, 2020.
...L-cysteine hydrochloride or hydrochloride monohydrate. Human hair may not be used as a source for this substance
- ^ "Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products". Retrieved July 28, 2021.
...ANNEX II LIST OF SUBSTANCES PROHIBITED IN COSMETIC PRODUCTS...416 Cells, tissues or products of human origin
- ^ "Questions About Food Ingredients: What is L-cysteine/cysteine/cystine?". Vegetarian Resource Group.
- ^ a b Drauz, Karlheinz; Grayson, Ian; Kleemann, Axel; Krimmer, Hans-Peter; Leuchtenberger, Wolfgang; Weckbecker, Christoph (2007). "Amino Acids". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a02_057.pub2. ISBN 978-3-527-30673-2.
- ^ Martens, Jürgen; Offermanns, Heribert; Scherberich, Paul (1981). "Facile Synthesis of Racemic Cysteine". Angewandte Chemie International Edition in English. 20 (8): 668. doi:10.1002/anie.198106681.
- ^ Hell R (1997). "Molecular physiology of plant sulfur metabolism". Planta. 202 (2): 138–48. Bibcode:1997Plant.202..138H. doi:10.1007/s004250050112. PMID 9202491. S2CID 2539629.
- ^ Bulaj G, Kortemme T, Goldenberg DP (June 1998). "Ionization-reactivity relationships for cysteine thiols in polypeptides". Biochemistry. 37 (25): 8965–72. doi:10.1021/bi973101r. PMID 9636038.
- ^ Sekhar, Rajagopal V; Patel, Sanjeet G (2011). "Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation". The American Journal of Clinical Nutrition. 94 (3): 847–853. doi:10.3945/ajcn.110.003483. PMC 3155927. PMID 21795440.

- ^ Lill R, Mühlenhoff U (2006). "Iron-sulfur protein biogenesis in eukaryotes: components and mechanisms". Annu. Rev. Cell Dev. Biol. 22: 457–86. doi:10.1146/annurev.cellbio.22.010305.104538. PMID 16824008.
- ^ Lippard, Stephen J.; Berg, Jeremy M. (1994). Principles of Bioinorganic Chemistry. Mill Valley, CA: University Science Books. ISBN 978-0-935702-73-6.[page needed]
- ^ Baker DH, Czarnecki-Maulden GL (June 1987). "Pharmacologic role of cysteine in ameliorating or exacerbating mineral toxicities". J. Nutr. 117 (6): 1003–10. doi:10.1093/jn/117.6.1003. PMID 3298579.
- ^ Heitmann P (January 1968). "A model for sulfhydryl groups in proteins. Hydrophobic interactions of the cystein side chain in micelles". Eur. J. Biochem. 3 (3): 346–50. doi:10.1111/j.1432-1033.1968.tb19535.x. PMID 5650851.
- ^ "A Review of Amino Acids (tutorial)". Curtin University. Archived from the original on 2015-09-07. Retrieved 2015-09-09.
- ^ Nagano N, Ota M, Nishikawa K (September 1999). "Strong hydrophobic nature of cysteine residues in proteins". FEBS Lett. 458 (1): 69–71. Bibcode:1999FEBSL.458...69N. doi:10.1016/S0014-5793(99)01122-9. PMID 10518936. S2CID 34980474.
- ^ Betts, M.J.; R.B. Russell (2003). "Hydrophobic amino acids". Amino Acid Properties and Consequences of Substitutions, In: Bioinformatics for Geneticists. Wiley. Retrieved 2012-09-16.
- ^ Gorga, Frank R. (1998–2001). "Introduction to Protein Structure--Non-Polar Amino Acids". Archived from the original on 2012-09-05. Retrieved 2012-09-16.
- ^ "Virtual Chembook--Amino Acid Structure". Elmhurst College. Archived from the original on 2012-10-02. Retrieved 2012-09-16.
- ^ Sevier CS, Kaiser CA (November 2002). "Formation and transfer of disulphide bonds in living cells". Nat. Rev. Mol. Cell Biol. 3 (11): 836–47. doi:10.1038/nrm954. PMID 12415301. S2CID 2885059.
- ^ Osawa, S; Jukes, T H; Watanabe, K; Muto, A (March 1992). "Recent evidence for evolution of the genetic code". Microbiological Reviews. 56 (1): 229–264. doi:10.1128/mr.56.1.229-264.1992. ISSN 0146-0749. PMC 372862. PMID 1579111.
- ^ Trifonov, Edward N. (September 2009). "The origin of the genetic code and of the earliest oligopeptides". Research in Microbiology. 160 (7): 481–486. doi:10.1016/j.resmic.2009.05.004. PMID 19524038.
- ^ Paulsen, Candice E.; Carroll, Kate S. (2013-07-10). "Cysteine-Mediated Redox Signaling: Chemistry, Biology, and Tools for Discovery". Chemical Reviews. 113 (7): 4633–4679. doi:10.1021/cr300163e. ISSN 0009-2665. PMC 4303468. PMID 23514336.
- ^ Giles, Niroshini M; Watts, Aaron B; Giles, Gregory I; Fry, Fiona H; Littlechild, Jennifer A; Jacob, Claus (August 2003). "Metal and Redox Modulation of Cysteine Protein Function". Chemistry & Biology. 10 (8): 677–693. doi:10.1016/s1074-5521(03)00174-1. ISSN 1074-5521. PMID 12954327.
- ^ Moosmann, Bernd; Behl, Christian (February 2008). "Mitochondrially encoded cysteine predicts animal lifespan". Aging Cell. 7 (1): 32–46. doi:10.1111/j.1474-9726.2007.00349.x. ISSN 1474-9718. PMID 18028257.
- ^ Sohal, Rajindar S (2002-07-01). "Role of oxidative stress and protein oxidation in the aging process1, 2". Free Radical Biology and Medicine. 33 (1): 37–44. doi:10.1016/S0891-5849(02)00856-0. ISSN 0891-5849. PMID 12086680.
- ^ Jacob, Claus; Giles, Gregory I.; Giles, Niroshini M.; Sies, Helmut (2003-10-13). "Sulfur and Selenium: The Role of Oxidation State in Protein Structure and Function". Angewandte Chemie International Edition. 42 (39): 4742–4758. doi:10.1002/anie.200300573. ISSN 1433-7851. PMID 14562341.
- ^ Nauser, Thomas; Pelling, Jill; Schöneich, Christian (2004-10-01). "Thiyl Radical Reaction with Amino Acid Side Chains: Rate Constants for Hydrogen Transfer and Relevance for Posttranslational Protein Modification". Chemical Research in Toxicology. 17 (10): 1323–1328. doi:10.1021/tx049856y. ISSN 0893-228X. PMID 15487892.
- ^ Moosmann, Bernd; Hajieva, Parvana; Behl, Christian (2006), "The antioxidant function of protein methionine explains about the evolution of a non-standard genetic code in mitochondria", Free Radical Biology and Medicine, 41 402: S149–S150, doi:10.1016/j.freeradbiomed.2006.10.015
- ^ Huang, Tzou-Chi; Ho, Chi-Tang (2001-07-27). Hui, Y. H.; Nip, Wai-Kit; Rogers, Robert (eds.). Meat Science and Applications, ch. Flavors of Meat Products. CRC. pp. 71–102. ISBN 978-0-203-90808-2.
- ^ "Food Ingredients and Colors". U.S. Food and Drug Administration. November 2004. Archived from the original on 2009-05-12. Retrieved 2009-09-06.
- ^ Otoyama, Ippo; Hamada, Hironobu; Kimura, Tatsushi; Namba, Haruchi; Sekikawa, Kiyokazu; Kamikawa, Norimichi; Kajiwara, Teruki; Aizawa, Fumiya; Sato, Yoshinobu M. (2019). "L-cysteine improves blood fluidity impaired by acetaldehyde: In vitro evaluation". PLOS ONE. 14 (3): e0214585. Bibcode:2019PLoSO..1414585O. doi:10.1371/journal.pone.0214585. PMC 6440629. PMID 30925182.
- ^ Salaspuro, Ville (2006). Interaction of alcohol and smoking in the pathogenesis of upper digestive tract cancers: possible chemoprevention with cysteine (Academic dissertation). University of Helsinki. pp. 41–44. hdl:10138/22689. URN:ISBN:952-10-3056-9.
- ^ Sprince H, Parker CM, Smith GG, Gonzales LJ (April 1974). "Protection against acetaldehyde toxicity in the rat by L-cysteine, thiamin and L-2-methylthiazolidine-4-carboxylic acid". Agents Actions. 4 (2): 125–30. doi:10.1007/BF01966822. PMID 4842541. S2CID 5924137.
- ^ Eriksson, C J Peter; Metsälä, Markus; Möykkynen, Tommi; Mäkisalo, Heikki; Kärkkäinen, Olli; Palmén, Maria; Salminen, Joonas E; Kauhanen, Jussi (20 October 2020). "L-Cysteine Containing Vitamin Supplement Which Prevents or Alleviates Alcohol-related Hangover Symptoms: Nausea, Headache, Stress and Anxiety". Alcohol and Alcoholism. 55 (6): 660–666. doi:10.1093/alcalc/agaa082. hdl:10138/339340. PMID 32808029.
- ^ Kanter MZ (October 2006). "Comparison of oral and i.v. acetylcysteine in the treatment of acetaminophen poisoning". Am J Health Syst Pharm. 63 (19): 1821–7. doi:10.2146/ajhp060050. PMID 16990628. S2CID 9209528.
- ^ Powell BC, Walker SK, Bawden CS, Sivaprasad AV, Rogers GE (1994). "Transgenic sheep and wool growth: possibilities and current status". Reprod. Fertil. Dev. 6 (5): 615–23. doi:10.1071/RD9940615. PMID 7569041.
- ^ Milkowski, John D.; Veber, Daniel F.; Hirschmann, Ralph (1979). "Thiol Protection with the Acetamidomethyl Group: S-Acetamidomethyl-L-Cysteine Hydrochloride". Organic Syntheses. 59: 190. doi:10.15227/orgsyn.059.0190.
- ^ Arnold, Alan P.; Jackson, W. Gregory (1990). "Stereospecificity in the Synthesis of the Tris((R)-Cysteinato-N,S)- and Tris((R)-Cysteinesulfinato-N,S)cobaltate(III) Ions". Inorganic Chemistry. 29 (18): 3618–3620. doi:10.1021/ic00343a061.
- ^ Anderson, Mary E.; Meister, Alton (1987). "Intracellular delivery of cysteine". Sulfur and Sulfur Amino Acids. Methods in Enzymology. Vol. 143. pp. 313–325. doi:10.1016/0076-6879(87)43059-0. ISBN 9780121820435. PMID 3309557.
- ^ Baumann, E. (1884). "Ueber Cystin und Cysteïn" [On cystine and cysteine]. Zeitschrift für physiologische Chemie (in German). 8: 299–305. From pp. 301-302: "Die Analyse der Substanz ergibt Werthe, welche den vom Cystin (C6H12N2S2O4) verlangten sich nähern, […] nenne ich dieses Reduktionsprodukt des Cystins: Cysteïn." (Analysis of the substance [cysteine] reveals values which approximate those [that are] required by cystine (C6H12N2S2O4), however the new base [cysteine] can clearly be recognized as a reduction product of cystine, to which the [empirical] formula C3H7NSO2, [which had] previously [been] ascribed to cystine, is [now] ascribed. In order to indicate the relationships of this substance to cystine, I name this reduction product of cystine: "cysteïne".) Note: Baumann's proposed structures for cysteine and cystine (see p.302) are incorrect: for cysteine, he proposed CH3CNH2(SH)COOH .
Further reading
[edit]- Nagano N, Ota M, Nishikawa K (September 1999). "Strong hydrophobic nature of cysteine residues in proteins". FEBS Lett. 458 (1): 69–71. Bibcode:1999FEBSL.458...69N. doi:10.1016/S0014-5793(99)01122-9. PMID 10518936. S2CID 34980474.
External links
[edit]- Holly (2005). Cystinuria Clearinghouse
- Cysteine MS Spectrum
- International Kidney Stone Institute Archived 2019-05-13 at the Wayback Machine