K-complex
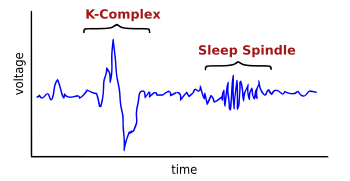
A K-complex is a waveform that may be seen on an electroencephalogram (EEG). It occurs during stage 2 NREM sleep. It is the "largest event in healthy human EEG".[1] They are more frequent in the first sleep cycles.
K-complexes have two proposed functions:[1] first, suppressing cortical arousal in response to stimuli that the sleeping brain evaluates not to signal danger, and second, aiding sleep-based memory consolidation.
The K-complex was discovered in 1937 in the private laboratories of Alfred Lee Loomis.[2]
Neurophysiology
[edit]K-complex consists of a brief negative high-voltage peak, usually greater than 100 μV, followed by a slower positive complex around 350 and 550 ms and at 900 ms a final negative peak. K-complexes occur roughly every 1.0–1.7 minutes and are often followed by bursts of sleep spindles. They occur spontaneously[1] but also occur in response to external stimuli such as sounds, and touches on the skin[3] and internal ones such as inspiratory interruptions.[4] They are generated in widespread cortical locations[1] though they tend to predominate over the frontal parts of the brain.[5]
Both K-complex and delta wave activity in stage 2 sleep create slow-wave (0.8 Hz) and delta (1.6–4.0 Hz) oscillations. However, their topographical distribution is different, and the delta power of K-complexes is higher.[6]
They are created by the occurrence in widespread cortical areas of outward dendritic currents from the middle (III) to the upper (I) layers of the cerebral cortex. This is accompanied by a decrease in broadband EEG power including gamma wave activity. This produces "down-states" of neuronal silence in which neural network activity is reduced.[1] The activity of K-complexes is transferred to the thalamus where it synchronizes the thalamocortical network during sleep, producing sleep oscillations such as spindles and delta waves.[7] It has been observed that they are indeed identical in the "laminar distributions of transmembrane currents" to the slow waves of slow-wave sleep.[1]
K-complexes have been suggested both to protect sleep and also to engage in information processing, as they are both an essential part of the synchronization of NREM sleep, while they also respond to both internal and external stimuli in a reactive manner.[8] This would be consistent with a function in suppressing cortical arousal in response to stimuli that the brain needs to initially process in regard to whether it is dangerous or not.[1]
Another suggested function is aiding the activation homeostasis of synapses[9] and memory consolidation. The activation thresholds of cortical synapses become lowered during wakefulness as they process information, making them more responsive, and so need to be adjusted back to preserve their signal-to-noise ratio.[9] The down-state provided by K-complexes does this by reducing the strengths of synaptic connections that occur while an individual is awake.[1] Further, the recovery from the down-state they induce allows that "cortical firing 'reboots' in a systematic order" so that memory engrams encoded during neuronal firing can be "repeatedly practiced and thus consolidated".[1]
Development
[edit]They are present in the sleep of 5-month-old infants, and develop with age. Between 3 and 5 years of age a faster negative component appears and continues to increase until adolescence. Another change occurs in adults: before 30 years of age their frequency and amplitude are higher than in older people particularly those over 50 years of age.[10] This parallels the decrease in other components of sleep such as sleep spindle density and delta power.[10]
Clinical
[edit]Epilepsy
[edit]In individuals with idiopathic generalized epilepsy, K-complex induced synchronization can trigger spike-and-wave discharges. This tends to happen most between the shift between waking and NREM, and between NREM and REM sleep.[11] In autosomal dominant nocturnal frontal lobe epilepsy, K-complexes are almost invariably present at the start of seizures.[12]
Restless legs syndrome
[edit]Individuals with restless legs syndrome have increased numbers of K-complexes, which are associated with (and often precede) leg movements. Dopamine enhancing drugs such as L-DOPA that reduce leg movements do not reduce the K-complex suggesting that they are primary and the leg movements secondary to them. Failure of such drugs to reduce K-complexes in spite of reducing the leg movements has been suggested to be why patients after such treatment continue to complain of non-restorative sleep.[13]
Obstructive sleep apnea
[edit]Obstructive sleep apnea syndrome is associated with inspiratory occlusions evoking fewer K-complexes during NREM sleep even though K-complexes are evoked normally to auditory stimuli and such individuals react normally to respiratory interruptions when awake. This suggests a link between such sleep apnea and a sleep specific blunted cortical response to respiratory problems.[14][15][16]
Notes
[edit]- ^ a b c d e f g h i Cash S.S.; Halgren E.; Dehghani N.; et al. (2009). "Human K-Complex Represents an Isolated Cortical Down-State". Science. 324 (5930): 1084–87. Bibcode:2009Sci...324.1084C. doi:10.1126/science.1169626. PMC 3715654. PMID 19461004.
- ^ Loomis A.L.; Harvey E.N.; Hobart G.A. (1937). "Cerebral states during sleep as studies by human brain potentials". J Exp Psychol. 21 (2): 127–44. doi:10.1037/h0057431.
- ^ Roth M.; Shaw J.; Green J. (1956). "The form, voltage distribution and physiological significance of the K-complex". Electroenceph Clin Neurophysiol. 8 (3): 385–402. doi:10.1016/0013-4694(56)90004-9. PMID 13330651.
- ^ Webster K.E.; Colrain I.M. (1998). "Multichannel EEG analysis of respiratory evoked-potential components during wakefulness and NREM sleep". J Appl Physiol. 85 (5): 1727–35. doi:10.1152/jappl.1998.85.5.1727. PMID 9804575.
- ^ McCormick L, Nielsen T, Nicolas A, Ptito M, Montplaisir J (1997). "Topographical distribution of spindles and K-complexes in normal subjects". Sleep. 20 (11): 939–41. doi:10.1093/sleep/20.11.939. PMID 9456457.
- ^ Happe S.; Anderer P.; Gruber G.; Klösch G.; Saletu B.; Zeitlhofer J. (2002). "Scalp topography of the spontaneous K-complex and of delta-waves in human sleep". Brain Topogr. 15 (1): 43–9. doi:10.1023/A:1019992523246. PMID 12371676. S2CID 21921834.
- ^ Amzica F.; Steriade M. (1998). "Cellular substrates and laminar profile of sleep K-complex". Neuroscience. 82 (3): 671–86. doi:10.1016/s0306-4522(97)00319-9. PMID 9483527. S2CID 26975545.
- ^ Halász P (2005). "K-complex, a reactive EEG graphoelement of NREM sleep: an old chap in a new garment". Sleep Med. Rev. 9 (5): 391–412. doi:10.1016/j.smrv.2005.04.003. PMID 16122950.
- ^ a b Tononi G.; Cirelli C. (2006). "Sleep function and synaptic homeostasis". Sleep Med. Rev. 10 (1): 49–62. doi:10.1016/j.smrv.2005.05.002. PMID 16376591.
- ^ a b Wauquier A (October 1993). "Aging and changes in phasic events during sleep". Physiol. Behav. 54 (4): 803–6. doi:10.1016/0031-9384(93)90095-w. PMID 8248360. S2CID 7736698.
- ^ Steriade M.; Amzica F. (1998). "Slow sleep oscillation, rhythmic K-complexes, and their paroxysmal developments". J Sleep Res. 7 (S1): 30–5. doi:10.1046/j.1365-2869.7.s1.4.x. PMID 9682191.
- ^ El Helou J.; Navarro V.; Depienne C.; Fedirko E.; LeGuern E.; Baulac M.; An-Gourfinkel I.; Adam C. (2008). "K-complex-induced seizures in autosomal dominant nocturnal frontal lobe epilepsy". Clin Neurophysiol. 119 (10): 2201–4. doi:10.1016/j.clinph.2008.07.212. PMID 18762450. S2CID 26640365.
- ^ Montplaisir J.; Boucher S.; Gosselin A.; Poirier G.; Lavigne G. (1996). "Persistence of repetitive EEG arousals (K-alpha complexes) in RLS patients treated with L-DOPA". Sleep. 19 (3): 196–9. doi:10.1093/sleep/19.3.196. PMID 8723375.
- ^ Huang J.; Colrain I.M.; Melendres M.C.; Karamessinis L.R.; Pepe M.E.; Samuel J.M.; Abi-Raad R.F.; Trescher W.H.; Marcus C.L. (2008). "Cortical processing of respiratory afferent stimuli during sleep in children with the obstructive sleep apnea syndrome". Sleep. 31 (3): 403–10. doi:10.1093/sleep/31.3.403. PMC 2276751. PMID 18363317.
- ^ Gora J, Trinder J, Pierce R, Colrain IM (November 2002). "Evidence of a sleep-specific blunted cortical response to inspiratory occlusions in mild obstructive sleep apnea syndrome". Am. J. Respir. Crit. Care Med. 166 (9): 1225–34. doi:10.1164/rccm.2106005. PMID 12403692.
- ^ Afifi L.; Guilleminault C.; Colrain I.M. (2003). "Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS". Respir Physiol Neurobiol. 136 (2–3): 221–34. doi:10.1016/s1569-9048(03)00084-3. PMID 12853013. S2CID 34199792.
