Immunogold labelling
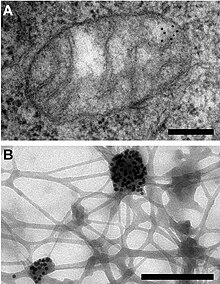
Immunogold labeling or immunogold staining (IGS) is a staining technique used in electron microscopy.[2] This staining technique is an equivalent of the indirect immunofluorescence technique for visible light. Colloidal gold particles are most often attached to secondary antibodies which are in turn attached to primary antibodies designed to bind a specific antigen or other cell component. Gold is used for its high electron density which increases electron scatter to give high contrast 'dark spots'.[3]
First used in 1971, immunogold labeling has been applied to both transmission electron microscopy and scanning electron microscopy, as well as brightfield microscopy. The labeling technique can be adapted to distinguish multiple objects by using differently-sized gold particles.
Immunogold labeling can introduce artifacts, as the gold particles reside some distance from the labelled object and very thin sectioning is required during sample preparation.[3]
History
[edit]Immunogold labeling was first used in 1971 by Faulk and Taylor to identify Salmonella antigens.[2][4] It was first applied in transmission electron microscopy (TEM) and was especially useful in highlighting proteins found in low densities, such as some cell surface antigens.[5] As the resolution of scanning electron microscopy (SEM) increased, so too did the need for nanoparticle-sized labels such as immunogold. In 1975, Horisberger and coworkers successfully visualised gold nanoparticles with a diameter of less than 30 nm[6] and this soon became an established SEM technique.[5]
Technique
[edit]First, a thin section of the sample is cut, often using a microtome.[7] Various other stages of sample preparation may then take place.
The prepared sample is then incubated with a specific antibody designed to bind the molecule of interest.[3] Next, a secondary antibody which has gold particles attached is added, and it binds to the primary antibody. Gold can also be attached to protein A or protein G instead of a secondary antibody, as these proteins bind mammalian IgG Fc regions in a non-specific way.[6]
The electron-dense gold particle can now be seen under an electron microscope as a black dot, indirectly labeling the molecule of interest.[3]
Labeling multiple objects
[edit]Immunogold labeling can be used to visualize more than one target simultaneously. This can be achieved in electron microscopy by using two different-sized gold particles.[8] An extension of this method used three different sized gold particles to track the localisation of regulatory peptides.[9] A more complex method of multi-site labeling involves labeling opposite sides of an antigenic site separately, the immunogold particles attached to both sides can then be viewed simultaneously.[10]
Uses in brightfield microscopy
[edit]Although immunogold labeling is typically used for transmission electron microscopy, when the gold is 'silver-enhanced' it can be seen using brightfield microscopy.[11] The silver enhancement increases the particle size, also making scanning electron microscopy possible. In order to produce the silver-enhanced gold particles, colloidal gold particles are placed in an acidic enhancing solution containing silver ions. Gold particles then act as a nucleation site and silver is deposited onto the particle. An example of the application of silver-enhanced immunogold labeling (IGSS) was in the identification of the pathogen Erwinia amylovora.[11]
Limitations
[edit]An inherent limitation to the immunogold technique is that the gold particle is around 15-30 nm away from the site to which the primary antibody is bound[5] (when using a primary and secondary antibodies labeling strategy). The precise location of the targeted molecule can therefore not be accurately calculated. Gold particles can be created with a diameter of 1 nm (or lower) but another limitation is then realized—at these sizes the gold label becomes hard to distinguish from tissue structure.[2][5]
Thin sections are required for immunogold labeling and these can produce misleading images; a thin slice of a cell component may not give an accurate view of its three-dimensional structure. For example, a microtubule may appear as a 'spike' depending on which plane the sectioning occurred. To overcome this limitation serial sections can be taken, which can then be compiled into a three-dimensional image.[3]
A further limitation is that antibodies and gold particles cannot penetrate the resin used to embed samples for imaging. Thus, only accessible molecules can be targeted and visualized. Labeling prior to embedding the sample can reduce the negative impact of this limitation.[3]
See also
[edit]References
[edit]- ^ Iborra FJ, Kimura H, Cook PR (2004). "The functional organization of mitochondrial genomes in human cells". BMC Biol. 2: 9. doi:10.1186/1741-7007-2-9. PMC 425603. PMID 15157274.
- ^ a b c "Immunogold Labeling in Scanning Electron Microscopy". Archived from the original on 2014-02-06. Retrieved 2010-07-08.
- ^ a b c d e f Alberts, Bruce; et al. (2008). Molecular biology of the cell (5th ed.). New York: Garland Science. ISBN 978-0-8153-4106-2.
- ^ Faulk WP, Taylor GM (November 1971). "An immunocolloid method for the electron microscope". Immunochemistry. 8 (11): 1081–3. doi:10.1016/0019-2791(71)90496-4. PMID 4110101.
- ^ a b c d Hermann R, Walther P, Müller M (1996). "Immunogold labeling in scanning electron microscopy". Histochemistry and Cell Biology. 106 (1): 31–39. doi:10.1007/BF02473200. PMID 8858365.
- ^ a b Roth J, Bendayan M, Orci L (December 1978). "Ultrastructural localization of intracellular antigens by the use of protein A-gold complex". J. Histochem. Cytochem. 26 (12): 1074–81. doi:10.1177/26.12.366014. PMID 366014.
- ^ Porter, K; Blum, J (1953). "A study in Microtomy for Electron Microscopy". The Anatomical Record. 117 (4): 685–710. doi:10.1002/ar.1091170403. PMID 13124776.
- ^ Roth J, Binder M (March 1978). "Coloidal gold, ferritin and peroxidase as markers for electron microscopic double labeling lectin techniques". J. Histochem. Cytochem. 26 (3): 163–9. doi:10.1177/26.3.632554. PMID 632554.
- ^ Tapia FJ, Varndell IM, Probert L, De Mey J, Polak JM (July 1983). "Double immunogold staining method for the simultaneous ultrastructural localization of regulatory peptides". J. Histochem. Cytochem. 31 (7): 977–81. doi:10.1177/31.7.6189888. PMID 6189888.
- ^ Bendayan M (January 1982). "Double immunocytochemical labeling applying the protein A-gold technique". J. Histochem. Cytochem. 30 (1): 81–5. doi:10.1177/30.1.6172469. PMID 6172469.
- ^ a b Van Laere O, De Wael L, De Mey J (1985). "Immuno gold staining (IGS) and immuno gold silver staining (IGSS) for the identification of the plant pathogenic bacterium Erwinia amylovora (Burrill) Winslow et al". Histochemistry. 83 (5): 397–9. doi:10.1007/BF00509198. PMID 2416717.
