Hyperandrogenism
| Hyperandrogenism | |
|---|---|
| Other names | Androgen excess |
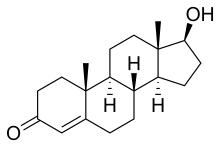 | |
| High levels of testosterone cause hyperandrogenism | |
| Pronunciation | |
| Specialty | Endocrinology |
| Symptoms | Acne, hair loss on scalp, increased body or facial hair, hypertension, infrequent or absent menstruation[1][2] |
| Causes | Polycystic ovary syndrome, adrenal hyperplasia, Cushing's disease, cancer[1][3] |
| Diagnostic method | Blood tests, ultrasound[1][4] |
| Treatment | Birth control pills, cyproterone acetate, spironolactone, antiandrogen[1] |
| Frequency | 5% in reproductive age women[2] |
Hyperandrogenism is a medical condition characterized by high levels of androgens. It is more common in women than men.[4] Symptoms of hyperandrogenism may include acne, seborrhea, hair loss on the scalp, increased body or facial hair, and infrequent or absent menstruation.[1][2] Complications may include high blood cholesterol and diabetes.[4] It occurs in approximately 5% of women of reproductive age.[2]
Polycystic ovary syndrome accounts for about 70% of hyperandrogenism cases.[1] Other causes include Congenital adrenal hyperplasia, insulin resistance, hyperprolactinemia, Cushing's disease, certain types of cancers, and certain medications.[4][1][3] Diagnosis often involves blood tests for testosterone, 17-hydroxyprogesterone, and prolactin, as well as a pelvic ultrasound.[1][4]
Treatment depends on the underlying cause.[4] Symptoms of hyperandrogenism can be treated with birth control pills or antiandrogens, such as cyproterone acetate or spironolactone.[1][4] Other measures may include hair removal techniques.[3]
The earliest known description of the condition is attributed to Hippocrates.[5][6]
In 2011, the International Association of Athletics Federations (now World Athletics) and IOC (International Olympic Committee)[7] released statements restricting the eligibility of female athletes with high testosterone, whether through hyperandrogenism or as a result of a difference in sex development (DSD). These regulations were referred to by both bodies as hyperandrogenism regulations and have led to athletes with DSDs being described as having hyperandrogenism.[8][9] They were revised in 2019 to focus more specifically on DSDs.[10]
Signs and symptoms
[edit]
Hyperandrogenism affects 5–10% of women of reproductive age.[11] Hyperandrogenism can affect both men and women but is more noticeable in women since elevated levels of androgens in women may facilitate virilization. Because hyperandrogenism is characterized by elevated male sex hormone levels, symptoms of hyperandrogenism in men are often negligible. Hyperandrogenism in women is typically diagnosed in late adolescence with a medical evaluation. The medical evaluation usually consists of a pelvic exam, observation of external symptoms, and a blood test measuring androgen levels.[12] Symptoms may include the following:
- Hirsutism (male-pattern hair growth)
- Alopecia (balding)
- Masculine appearance
- Hidradenitis suppurativa
- Polycystic ovarian syndrome
- Oligomenorrhea (menstrual irregularities)
- Acne
- Obesity
- Infertility
- Deepening of voice
- Oily skin
- Seborrhea (skin inflammation)
- Libido (increased sex drive)
- Type 2 diabetes
Women
[edit]Hyperandrogenism, especially high levels of testosterone, can cause serious adverse effects if left untreated. High testosterone levels are associated with other health conditions such as obesity, hypertension, amenorrhea (cessation of menstrual cycles), and ovulatory dysfunction, which can lead to infertility. Prominent signs of hyperandrogenism are hirsutism (unwanted growth of hair, especially in the abdominal region and on the back), adult acne, deepening of the voice, and alopecia (balding).[13]
Hyperandrogenism has also been observed to increase insulin tolerance, which can lead to type two diabetes and dyslipidemia, such as high cholesterol. These effects may have psychological impacts, sometimes leading to social anxiety and depression, especially in adolescent girls and young women. Paired with obesity and hirsutism, it can cause the individual to have low self-esteem.[12][14]
Men
[edit]Administration of high-dose testosterone in men over a course of weeks can cause an increase in aggression and hypomanic symptoms, though these were seen in only a minority of subjects.[15] Acute high-dose anabolic-androgenic steroid administration in males attenuates endogenous sex hormone production and affects the thyroid hormone axis. Effects on mood and aggression observed during high-dose anabolic-androgenic steroid administration may occur secondarily to hormonal changes.[16] Many of the same signs and symptoms that are seen in women, such as alopecia and acne, may also be found in men.[17] Enlargement of the prostate may also occur.[17]
Causes
[edit]While hyperandrogenism in women can be caused by external factors, it can also appear spontaneously.
Polycystic ovary syndrome
[edit]
Polycystic ovary syndrome (PCOS) is an endocrine disorder characterized by an excess of androgens produced by the ovaries. It is estimated that approximately 90% of women with PCOS demonstrate hypersecretion of these hormones.[18] The cause of this condition is unknown. Speculations include genetic predisposition; however, the gene or genes responsible for this remain unidentified.[19] The condition may have a hereditary basis. Other possible causes include elevated insulin production. Most cases of PCOS involve insulin resistance.[20] It is thought that adipose tissue dysfunction plays a role in the insulin resistance seen in PCOS.[20] Insulin can induce excess testosterone secretion from the ovaries.[21] A complication associated with polycystic ovary syndrome is high cholesterol, which is treated with statins. In a meta-analysis, atorvastatin was shown to decrease androgen concentrations in people with hyperandrogenism.[22]
Elevated insulin leads to lower production of sex hormone binding globulin (SHBG), a regulatory glycoprotein that suppresses the function of androgens.[23] High blood levels of insulin also work in conjunction with ovarian sensitivity to insulin to cause hyperandrogenemia, the primary symptom of PCOS. Obese individuals may be more biologically inclined to PCOS due to markedly higher insulin. This hormonal imbalance can lead to chronic anovulation, in which the ovaries fail to release mature eggs. These cases of ovulatory dysfunction are linked to infertility and menstrual disturbances.[18][24] A post hoc analysis from a randomized, placebo-controlled, multi-centre study carried out at 11 secondary care centres, as well as a longitudinal single-centre study on pregnant women in Norway, also determined that metformin had no effect on maternal androgens in pregnancies occurring in the setting of PCOS.[25]
One systemic review suggested that polymorphisms in the vitamin D receptor gene are associated with the prognosis of polycystic ovary syndrome, though this is based on small sample sizes and is debated.[26][27] Studies have shown benefits for vitamin D supplementation in women with vitamin D deficiency and PCOS.[28]
Hyperinsulinemia can increase the production of androgens in the ovaries.[29] One context in which this occurs is HAIR-AN syndrome, a rare subtype of PCOS.[30][31]
Hyperthecosis and hyperinsulinemia
[edit]Hyperthecosis occurs when the cells of the ovarian stroma transition from interstitial cells, located between other cells, into luteinized theca cells. Theca cells are located in the ovarian follicles and become luteinized when the ovarian follicle bursts and a new corpus luteum is formed. The dispersal of luteinized theca cells throughout the ovarian stroma—in contrast to their distribution in PCOS, in which luteinized theca cells occur around cystic follicles only—causes women with hyperthecosis to have higher testosterone levels and virilization than women with PCOS. Elevated insulin is also characteristic of hyperthecosis.[32] Hyperthecosis most commonly develops in postmenopausal women and is linked to acne, hirsutism, growth of the clitoris, baldness, and voice deepening.[33]
Obesity can play a role in insulin resistance.[34] It makes thecal cells more responsive to luteinizing hormone.[34] Therefore, obesity increases ovarian androgen production.[34] Additionally, obesity elevates inflammatory adipokines which leads to not only adipogenesis, but also heightened insulin resistance.[34]
Cushing's syndrome
[edit]Cushing's syndrome develops as a result of long-term exposure to the hormone cortisol.[35][36] Cushing's syndrome can either be exogenous or endogenous, depending on whether it is caused by an external or internal source, respectively.[37] The intake of glucocorticoids, a type of corticosteroid, is a common cause for the development of exogenous Cushing's syndrome. Endogenous Cushing's syndrome can occur when the body produces excess cortisol. This occurs when the hypothalamus of the brain signals to the pituitary gland with excess corticotropin-releasing hormone, which in turn secretes adrenocorticotropin hormone (ACTH). ACTH then causes the adrenal glands to release cortisol into the blood. Signs of Cushing's syndrome include muscle weakness, easy bruising, weight gain, male-pattern hair growth (hirsutism), coloured stretch marks, and an excessively reddish complexion in the face.[38] Cushing's syndrome can cause androgen excess and hence the signs and symptoms of hyperandrogenism.[33]
Congenital adrenal hyperplasia
[edit]Congenital adrenal hyperplasia (CAH) describes a group of autosomal recessive disorders that cause a lack of an enzyme necessary for the production of cortisol and/or aldosterone, steroid hormones produced by the adrenal cortex. Most cases of CAH are due to 21-hydroxylase deficiencies. The heightened androgen levels seen in congenital adrenal hyperplasia affect the hypothalamic–pituitary–gonadal axis.[39] Heightened androgen levels can also affect the ovaries, which can lead to infertility as well as chronic anovulation.[39]
Since CAH consists of multiple disorders, the signs, symptoms and severity of hyperandrogenism may stem from a variety of specific mutations.[40] Genotyping is therefore critical to verify diagnoses and to establish prognostic factors for individuals.[41] Genotyping is also crucial for people seeking to use genetic counselling as an aid to family planning.[41]
In women, CAH causes ambiguous genitals at birth and excessive pubic hair, enlargement of the clitoris, and hirsutism in adolescence. Although CAH causes rapid growth in childhood, adult women with CAH are shorter than average due to early puberty and closure of the growth plates in the long bones. Symptoms in males include early showings of pubic hair, enlargement of the penis, and rapid musculoskeletal growth.[42]
Tumors
[edit]Adrenocortical carcinoma and tumors
[edit]Adrenocortical carcinoma occurs rarely; the average incidence rate is estimated to be 1–2 cases per million annually.[43] The disease involves the formation of cancerous cells within the cortex of one or both of the adrenal glands. Although these tumors are identified in fewer than two percent of patients diagnosed with hyperandrogenism, the possibility must be considered within this population. In one study, more than half of tumor-affected patients had elevated levels of the androgens androstenedione, dehydroepiandrosterone sulfate, and testosterone.[44] The elevation of androgens caused by adrenocortical carcinomas often causes patients to develop Cushing's syndrome, primary aldosteronism, and hyperandrogenism.[45][44] The molecular basis of the disease has yet to be elucidated.[44]
Adenoma of the adrenal gland
[edit]Adrenal adenomas are benign tumors of the adrenal gland. In most cases, the tumors display no symptoms and require no treatment. In rare cases, however, some adrenal adenomas may become activated. When activated, the adenoma begins to produce hormones in much larger quantities than what the adrenal glands would normally produce, leading to health complications including primary aldosteronism and hyperandrogenism.[46]
Arrhenoblastoma
[edit]Arrhenoblastoma is an uncommon tumor of the ovary. It is composed of sterol cells, Leydig cells, or some combination of the two. The tumor can produce male or female hormones and may cause masculinization. In a prepubescent child, a tumor may cause precocious puberty. Malignant arrhenoblastoma accounts for 30% of cases of arrhenoblastoma, the other 70% being largely benign and curable with surgery.[47]
Hilar cell tumor
[edit]A hilar cell tumor is an androgen-producing ovarian tumor that is most commonly found in older women and often leads to the development of male sex characteristics. The tumor tends to occur around the region of the ovary where the blood vessels enter the organ, known as the hilum. This type of tumor tends to be small in size and in most cases can be entirely removed and its symptoms reversed through surgery.[48]
Krukenberg tumor
[edit]A Krukenberg tumor is a quickly developing malignant tumor found in one or both ovaries. In most cases, the tumor primarily originates from tissues in the stomach, pancreas, gallbladder, colon, or breast. It colonized the ovary by spreading through the peritoneal cavity.[49] These tumors cause virilization. Increased androgen production due to elevations in human chorionic gonadotropin is hypothesized as the main cause of hyperandrogenism in women with Krukenberg tumors.[50]
Menopause
[edit]The end of ovulation and the beginning of menopause can result in hyperandrogenism. During this transition, the body stops releasing estrogen at a faster rate than it stops releasing androgens. In some cases, the difference between the lower estrogen levels and higher androgen levels can produce hyperandrogenism. A decrease in sex hormone levels while the free androgen index increases can also contribute to this process.[51]
Drugs
[edit]Many drugs can provoke symptoms of hyperandrogenism. These symptoms include, but are not limited to hirsutism, acne, dermatitis, androgenic alopecia, irregularities in menstruation, clitoral hypertrophy, and the deepening of the voice. Drugs most frequently implicated in hyperandrogenism include anabolic steroids, synthetic progestins, and antiepileptics; however, many other drugs may also cause hyperandrogenism.[52] This can happen through one of five mechanisms: the direct introduction of androgens to the body, the binding of the drug to androgen receptors (as is the case with anabolic-androgenic steroids), a reduction of sex hormone-binding globulin plasma concentration that leads to an increase in free testosterone, interference with the hypothalamic–pituitary–ovarian (HPO) axis, or an increase in the release of adrenal androgens.[53] Certain drugs cause hyperandrogenism through mechanisms that remain unclear. For example, the molecular basis by which valproate induces hyperandrogenism and polycystic ovary syndrome has yet to be determined.[52] However, one study showed that women taking valproic acid had higher testosterone levels and incidences of hyperandrogenism compared to women who were not taking valproic acid.[54]
Heredity
[edit]Hyperandrogenism can appear as a symptom of many different genetic and medical conditions. Some of the conditions with hyperandrogenic symptoms, including PCOS, may sometimes be hereditary. Additionally, it is thought that epigenetics may contribute to the pathogenesis of polycystic ovary syndrome.[55]
One potential cause of PCOS is maternal hyperandrogenism, whereby hormonal irregularities in the mother can affect the development of the child during gestation, resulting in the passing of polycystic ovary syndrome from mother to child.[56] However, no androgen elevations were found in the umbilical cord blood of children born to mothers with PCOS.[57]
Diagnosis
[edit]Diagnosing hyperandrogenism can be complex due to the wide variety and severity of signs and symptoms that may present.[58] It is most often diagnosed by checking for signs of hirsutism according to a standardized method that scores the range of excess hair growth.[11][12]
Girls may show symptoms of hyperandrogenism early in life, but physicians become more concerned when the patient is in her late teens or older.[12]
Checking medical history and a physical examination of symptoms are used for an initial diagnosis.[12] Patient history assessed includes age at thelarche, adrenarche, and menarche; patterns of menstruation; obesity; reproductive history; and the start and advancement of hyperandrogenism symptoms.[12] Patterns of menstruation are examined since irregular patterns may accompany hyperandrogenism.[11] Other conditions that may present alongside hirsutism that can contribute to diagnosis include androgenic alopecia and acne.[58] If hyperandrogenism is severe, virilization may occur.[58]
Family history is also assessed for occurrences of hyperandrogenism symptoms or obesity in other family members.[12]
Laboratory tests can measure FSH, luteininzing hormone, DHEAS, prolactin, 17α-hydroxyprogesterone, and total and free testosterone in the blood.[12] Abnormally high levels of any of these hormones help in diagnosing hyperandrogenism.[12]
Prevention
[edit]Since risk factors are not known and vary among individuals with hyperandrogenism, there is no sure method to prevent the condition.[59] Accordingly, more long-term studies are needed to find a cause of the condition before a sufficient method of prevention can be established.[59]
Despite this, there are a few things that can help avoid long-term medical issues related to hyperandrogenism and PCOS. Getting checked by a medical professional for hyperandrogenism — especially if one has a family history of the condition, irregular periods, or diabetes — can be beneficial.[60] A healthy weight and diet may reduce the chances, as continued exercise and a healthy diet lead to an improved menstrual cycle, decreased insulin levels, and lowered androgen concentrations.[59]
Treatment
[edit]There is no definitive treatment for hyperandrogenism as it varies with the underlying condition that causes it. As a hormonal symptom of PCOS, menopause, and other endocrine conditions, it is primarily treated as a symptom of these conditions. Drugs may be considered only in women who do not plan on becoming pregnant in the near future.[61] Some effective drugs for facial hirsutism includes eflornithine, which may cause birth defects in pregnant women.[62] Retinoids and antibiotics can be used for acne and minoxidil for alopecia.[62] Systemically, it is treated with antiandrogens such as cyproterone acetate, flutamide and spironolactone to reduce androgenic signaling. For hyperandrogenism caused by late-onset congenital adrenal hyperplasia (LOCAH), treatment is primarily focused on providing the patient with glucocorticoids to combat the low cortisol production and the corresponding increase in androgens caused by the increase in size of the adrenal glands.[63][64] Estrogen-based oral contraceptives are used to treat both LOCAH- and PCOS-associated hyperandrogenism. These hormonal treatments reduce the androgen excess and suppress adrenal androgen production, bringing about a significant decrease in hirsutism.[65][66]
Hyperandrogenism is often managed symptomatically. Hirsutism and acne both respond well to the hormonal treatments described above, with 60–100% of patients reporting an improvement in hirsutism.[65] Androgenic alopecia however, does not show an improvement with hormonal treatments and requires other treatments, such as hair transplantation.[67]
Supplementation can also contribute to the managing the symptomatic effects of hyperandrogenism. In a meta-analysis, high-dose vitamin D supplements given to women with vitamin D deficiency due to PCOS improved glucose levels, insulin sensitivity, and cholesterol levels, as well as lowering testosterone, sex hormone-binding globulin, and the free androgen index, all of which are associated with hyperandrogenism.[68] Vitamin D supplementation in women with vitamin D deficiency but without PCOS did not show the same results.[28]
Targeting insulin resistance and obesity
[edit]Lifestyle modifications are the first-line treatment for PCOS.[69] They help improve body composition, insulin resistance, and hyperandrogenism. However, it is unclear whether they help improve mood, quality of life, and reproductive outcomes.[70] A meta-analysis study in 2017 showed that bariatric surgery in women with severe obesity and PCOS decreased levels of total and free testosterone and helped correct hirsutism and menstrual dysfunction.[71]
Insulin resistance in women with PCOS is typically treated with insulin-sensitizer drugs such as metformin. Metformin can help to decrease weight and androgen levels.[72] When combined with lifestyle modifications (changes in diet and exercise), it has been linked with lower body mass index and a reduction in menstrual problems.[72] However, the use of metformin in women with PCOS should only be considered in patients with impaired glucose tolerance.[73]
Society and culture
[edit]Because androgen excess is manifested in noticeable physical features (such as hirsutism), social stigma may be associated with it.[citation needed]
Sports
[edit]Current evidence-based studies show that unusually high levels of circulating testosterone are associated with increased athletic performance in women, unless they lack androgen sensitivity. However, controversy has emerged in the form of the claim that testosterone is not unlike any other physical parameter with reference to bestowing advantages or disadvantages on female athletes. Existing regulations throughout competitive sports are currently being refined to specifically address this particular claim.[74]

Following the case of South African athlete Caster Semenya, an athlete with a difference in sex development (DSD), the International Association of Athletics Federations introduced its hyperandrogenism regulations, which restricted women with high testosterone levels, whether the hormones were produced by ovaries, adrenals, or testes. These regulations replaced the earlier sex verification rules.[citation needed]
Following a series of legal challenges, regulations called the Eligibility Regulations for the Female Classification (Athletes with Differences of Sexual Development) were released on 1 May 2019.[10] These regulations apply only to athletes who have a DSD, high testosterone and virilization,[75] and no longer include hyperandrogenism from non-DSD-related causes such as PCOS. Such DSDs, often seen in people who have a Y chromosome and testes, include 5α‐reductase deficiency, partial androgen insensitivity, and congenital adrenal hyperplasia.[citation needed]
Social definition
[edit]Cultural variation can define hyperandrogenism socially—apart from clinical and chemical definitions—to make some hair growth unacceptable even if it is considered clinically normal based on metrics like the Ferriman-Gallwey score. For example, only pubic and axillary hair may be tolerated in North American women, while other androgen-dependent hair such as growth on the upper lip, over the linea alba, on the thighs, and around the areola is not.[76]
Organizations
[edit]Professional organizations such as the Androgen Excess and PCOS Society exist to promote the research, treatment, diagnosis, and prevention of such disorders and to educate the public and scientific community about them.[77]
InterACT, an intersex organization, listed hyperandrogenism as an intersex variation in a glossary from 2022.[78]
See also
[edit]- Hypoandrogenism
- Hypergonadism
- Hypergonadotropic hypergonadism
- Hypogonadism
- Hyperestrogenism
- Hypoestrogenism
- Androgen-dependent condition
References
[edit]- ^ a b c d e f g h i Peigné M, Villers-Capelle A, Robin G, Dewailly D (2013). "[Hyperandrogenism in women]". Presse Médicale. 42 (11): 1487–99. doi:10.1016/j.lpm.2013.07.016. PMID 24184282. S2CID 28921380.
- ^ a b c d Curtis M, Antoniewicz L, Linares ST (2014). Glass' Office Gynecology. Lippincott Williams & Wilkins. p. 39. ISBN 978-1-60831-820-9. Archived from the original on 24 February 2024. Retrieved 30 August 2020.
- ^ a b c Catteau-Jonard S, Cortet-Rudelli C, Richard-Proust C, Dewailly D (2012). "Hyperandrogenism in adolescent girls". Endocrine Development. 22: 181–193. doi:10.1159/000326688. ISBN 978-3-8055-9336-6. PMID 22846529.
- ^ a b c d e f g Carlson KJ, Eisenstat SA (2004). The New Harvard Guide to Women's Health. Harvard University Press. p. 286. ISBN 978-0-674-01282-0.
- ^ Banker M (2019). Nova IVI Textbook of Infertility & Assisted Reproductive Technology. JP Medical Ltd. p. 237. ISBN 978-9-3889-5884-4. Archived from the original on 24 February 2024. Retrieved 30 August 2020.
- ^ Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms. Elsevier. 2014. p. 1385. ISBN 978-0-12-386457-4. Archived from the original on 24 February 2024. Retrieved 30 August 2020.
- ^ "IOC addresses eligibility of female athletes with hyperandrogenism". International Olympic Committee. 2011. Archived from the original on 25 October 2020. Retrieved 20 September 2020.
- ^ Washington Post Staff (2019). "What are the issues behind the Court of Arbitration for Sport ruling in Caster Semenya case?". The Washington Post. Archived from the original on 25 October 2020. Retrieved 20 September 2020.
- ^ Abraham R (2019). "What's with the gender inequality? Dutee Chand talks about the tests female athletes face before competing". The Economic Times. Bennett, Coleman & Co. Ltd. Archived from the original on 15 December 2020. Retrieved 20 September 2020.
- ^ a b "Eligibility Regulations for the Female Classification (Athletes with Differences of Sex Development)". International Association of Athletics Federations. 1 May 2019. Archived from the original on 24 February 2024. Retrieved 20 September 2020.
- ^ a b c Yildiz BO (2006). "Diagnosis of hyperandrogenism: clinical criteria". Best Practice & Research. Clinical Endocrinology & Metabolism. 20 (2): 167–76. doi:10.1016/j.beem.2006.02.004. PMID 16772149.
- ^ a b c d e f g h i Goodman NF, Bledsoe MB, Cobin RH, Futterweit W, Goldzieher JW, Petak SM, et al. (2001). "American Association of Clinical Endocrinologists medical guidelines for the clinical practice for the diagnosis and treatment of hyperandrogenic disorders". Endocrine Practice. 7 (2): 120–34. doi:10.4158/EP.7.2.120. PMID 12940239.
- ^ Simon J (2015). "Androgen". Health Women. National Women's Health Resource Center. Archived from the original on 15 November 2016. Retrieved 14 November 2016.
- ^ Brettenthaler N, De Geyter C, Huber PR, Keller U (2004). "Effect of the insulin sensitizer pioglitazone on insulin resistance, hyperandrogenism, and ovulatory dysfunction in women with polycystic ovary syndrome". The Journal of Clinical Endocrinology and Metabolism. 89 (8) (published 28 April 2011): 3835–40. doi:10.1210/jc.2003-031737. PMID 15292314.
- ^ Pope HG, Kouri EM, Hudson JI (2000). "Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial". Archives of General Psychiatry. 57 (2): 133–40, discussion 155–6. doi:10.1001/archpsyc.57.2.133. PMID 10665615.
- ^ Daly, R.C.; Su, T.-P.; Schmidt, P.J.; Pagliaro, M.; Pickar, D.; Rubinow, D.R. (2003). "Neuroendocrine and behavioral effects of high-dose anabolic steroid administration in male normal volunteers". Psychoneuroendocrinology. 28 (3): 317–331. doi:10.1016/S0306-4530(02)00025-2. ISSN 0306-4530. PMID 12573299. S2CID 7170203. Archived from the original on 27 July 2021. Retrieved 27 July 2021.
- ^ a b See Table 1 in Singh, Garima; Magani, Sri Krishna Jayadev; Sharma, Rinku; Bhat, Basharat; Shrivastava, Ashish; Chinthakindi, Madhusudhan; Singh, Ashutosh (3 October 2019). "Structural, functional and molecular dynamics analysis of cathepsin B gene SNPs associated with tropical calcific pancreatitis, a rare disease of tropics". PeerJ. 7: e7425. doi:10.7717/peerj.7425. PMC 6778667. PMID 31592339.
- ^ a b Franks S (1995). "Polycystic ovary syndrome". The New England Journal of Medicine. 333 (13): 853–61. doi:10.1056/NEJM199509283331307. PMID 7651477.
- ^ "Polycystic Ovary Syndrome (PCOS)." Causes. Mayo Clinic, n.d. Web. 9 November 2016.
- ^ a b Goodarzi, Mark O.; Dumesic, Daniel A.; Chazenbalk, Gregorio; Azziz, Ricardo (2011). "Polycystic ovary syndrome: etiology, pathogenesis and diagnosis". Nature Reviews. Endocrinology. 7 (4): 219–231. doi:10.1038/nrendo.2010.217. ISSN 1759-5037. PMID 21263450. S2CID 205479927. Archived from the original on 30 July 2021. Retrieved 30 July 2021.
- ^ "Defining PCOS". The University of Chicago Medical Center. Archived from the original on 27 July 2021. Retrieved 27 July 2021.
- ^ Sathyapalan, Thozhukat; Smith, Karen A.; Coady, Anne-Marie; Kilpatrick, Eric S.; Atkin, Stephen L. (2012). "Atorvastatin therapy decreases androstenedione and dehydroepiandrosterone sulphate concentrations in patients with polycystic ovary syndrome: randomized controlled study". Annals of Clinical Biochemistry. 49 (Pt 1): 80–85. doi:10.1258/acb.2011.011071. ISSN 1758-1001. PMID 21972424.
- ^ Hammond GL, Bocchinfuso WP (1996). "Sex hormone-binding globulin: gene organization and structure/function analyses". Hormone Research. 45 (3–5): 197–201. doi:10.1159/000184787. PMID 8964583.
- ^ Burd I, Zieve D, Ogilvie I (27 January 2020). "Polycystic Ovary Syndrome". MedlinePlus Medical Encyclopedia. Archived from the original on 29 July 2021. Retrieved 27 July 2021.
- ^ Andræ F, Abbott D, Stridsklev S, Schmedes AV, Odsæter IH, Vanky E, Salvesen Ø (2020). "Sustained Maternal Hyperandrogenism During PCOS Pregnancy Reduced by Metformin in Non-obese Women Carrying a Male Fetus". The Journal of Clinical Endocrinology and Metabolism. 105 (12): 3762–3770. doi:10.1210/clinem/dgaa605. PMC 7538101. PMID 32866967.
- ^ Reis, Guilherme Victor Oliveira Pimenta Dos; Gontijo, Natália Alves; Rodrigues, Kathryna Fontana; Alves, Michelle Teodoro; Ferreira, Cláudia Natália; Gomes, Karina Braga (2017). "Vitamin D receptor polymorphisms and the polycystic ovary syndrome: A systematic review". The Journal of Obstetrics and Gynaecology Research. 43 (3): 436–446. doi:10.1111/jog.13250. ISSN 1447-0756. PMID 28127831. S2CID 11152805.
- ^ El-Shal, Amal S.; Shalaby, Sally M.; Aly, Nader M.; Rashad, Nearmeen M.; Abdelaziz, Ahmed M. (2013). "Genetic variation in the vitamin D receptor gene and vitamin D serum levels in Egyptian women with polycystic ovary syndrome". Molecular Biology Reports. 40 (11): 6063–6073. doi:10.1007/s11033-013-2716-y. ISSN 1573-4978. PMID 24078159. S2CID 18113257. Archived from the original on 29 July 2021. Retrieved 29 July 2021.
- ^ a b Karadağ, Cihan; Yoldemir, Tevfik; Yavuz, Dilek Gogas (2018). "Effects of vitamin D supplementation on insulin sensitivity and androgen levels in vitamin-D-deficient polycystic ovary syndrome patients". The Journal of Obstetrics and Gynaecology Research. 44 (2): 270–277. doi:10.1111/jog.13516. ISSN 1447-0756. PMID 29094433. S2CID 46784152. Archived from the original on 29 July 2021. Retrieved 29 July 2021.
- ^ Barbieri RL, Hornstein MD (December 1988). "Hyperinsulinemia and ovarian hyperandrogenism. Cause and effect". Endocrinology and Metabolism Clinics of North America. 17 (4): 685–703. doi:10.1016/S0889-8529(18)30405-5. PMID 3058472.
- ^ James W, Berger T, Elston D (2005). Andrews' Diseases of the Skin: Clinical Dermatology (10th ed.). Saunders. ISBN 978-0-8089-2351-0.
- ^ Somani N, Harrison S, Bergfeld WF (2008). "The clinical evaluation of hirsutism". Dermatologic Therapy. 21 (5): 376–91. doi:10.1111/j.1529-8019.2008.00219.x. PMID 18844715. S2CID 34029116.
- ^ Pasquali R (2011). "Research in Polycystic Ovary Syndrome Today and Tomorrow". Medscape. Blackwell Publishing. Archived from the original on 2 January 2017. Retrieved 14 November 2016.
- ^ a b Atmaca M, Seven İ, Üçler R, Alay M, Barut V, Dirik Y, Sezgin Y (16 December 2014). "An interesting cause of hyperandrogenemic hirsutism". Case Reports in Endocrinology. 2014: 987272. doi:10.1155/2014/987272. PMC 4280803. PMID 25580312.
- ^ a b c d Glueck, Charles J.; Goldenberg, Naila (2019). "Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics". Metabolism: Clinical and Experimental. 92: 108–120. doi:10.1016/j.metabol.2018.11.002. ISSN 1532-8600. PMID 30445140. S2CID 53567436. Archived from the original on 1 August 2021. Retrieved 1 August 2021.
- ^ "Cushing's Syndrome". National Endocrine and Metabolic Diseases Information Service (NEMDIS). July 2008. Archived from the original on 10 February 2015. Retrieved 16 March 2015.
These benign, or noncancerous, tumors of the pituitary gland secrete extra ACTH. Most people with the disorder have a single adenoma. This form of the syndrome, known as Cushing's disease
- ^ Forbis P (2005). Stedman's medical eponyms (2nd ed.). Baltimore, Md.: Lippincott Williams & Wilkins. p. 167. ISBN 978-0-7817-5443-9. Archived from the original on 24 February 2024. Retrieved 11 July 2021.
- ^ "Cushing's Syndrome". The Lecturio Medical Concept Library. Archived from the original on 22 September 2021. Retrieved 11 July 2021.
- ^ "Cushing's Syndrome". National Institute of Diabetes and Digestive and Kidney Diseases. April 2012. Archived from the original on 4 October 2016. Retrieved 14 November 2016.
- ^ a b Pignatelli, Duarte; Pereira, Sofia S.; Pasquali, Renato (2019). "Androgens in Congenital Adrenal Hyperplasia". Frontiers of Hormone Research. 53: 65–76. doi:10.1159/000494903. ISBN 978-3-318-06470-4. ISSN 1662-3762. PMID 31499506. S2CID 202412336. Archived from the original on 2 August 2021. Retrieved 2 August 2021.
- ^ Witchel, Selma Feldman (2017). "Congenital Adrenal Hyperplasia". Journal of Pediatric and Adolescent Gynecology. 30 (5): 520–534. doi:10.1016/j.jpag.2017.04.001. ISSN 1873-4332. PMC 5624825. PMID 28450075.
- ^ a b Török, Dóra (2019). "Congenital Adrenal Hyperplasia". Genetics of Endocrine Diseases and Syndromes. Experientia Supplementum. Vol. 111. pp. 245–260. doi:10.1007/978-3-030-25905-1_12. ISBN 978-3-030-25904-4. ISSN 1664-431X. PMID 31588535. S2CID 203849848. Archived from the original on 1 August 2021. Retrieved 1 August 2021.
{{cite book}}:|journal=ignored (help) - ^ Wilson T (23 June 2016). "Congenital Adrenal Hyperplasia". Medscape. Archived from the original on 15 November 2016. Retrieved 14 November 2016.
- ^ Martin Fassnacht, Bruno Allolio (June 2006). ""Adrenocortical carcinoma: clinical update." The Journal of Clinical Endocrinology & Metabolism 91.6 (2006): 2027-2037". The Journal of Clinical Endocrinology & Metabolism.
- ^ a b c Di Dalmazi, Guido (2019). "Hyperandrogenism and Adrenocortical Tumors". Frontiers of Hormone Research. 53: 92–99. doi:10.1159/000494905. ISBN 978-3-318-06470-4. ISSN 1662-3762. PMID 31499503. S2CID 202413714. Archived from the original on 27 July 2021. Retrieved 27 July 2021.
- ^ "Adrenocortical Carcinoma". National Cancer Institute. 27 February 2019. Archived from the original on 27 July 2021. Retrieved 27 July 2021.
- ^ "Adenoma of the Adrenal Gland". Genetic and Rare Diseases Information Center(GARD) – an NCATS Program. U.S National Library of Medicine. U.S. National Library of Medicine. 26 November 2014. Archived from the original on 27 July 2021. Retrieved 27 July 2021.
- ^ Martin E, ed. (2015). "Arrhenoblastoma". Concise medical dictionary. Oxford University Press. p. 11. ISBN 978-0-19-968799-2. Archived from the original on 24 February 2024. Retrieved 27 July 2021.
- ^ Martin E, ed. (2015). "Hilar cell tumour". Concise medical dictionary. Oxford University Press. p. 353. ISBN 978-0-19-968799-2.
- ^ Concise medical dictionary. E. A. Martin (8th ed.). [Oxford]: Oxford University Press. 2010. ISBN 978-0-19-172701-6. OCLC 894628585. Archived from the original on 24 February 2024. Retrieved 29 July 2021.
{{cite book}}: CS1 maint: others (link) - ^ Bustamante, Carmen; Hoyos-Martínez, Alfonso; Pirela, Daniela; Díaz, Alejandro (2017). "In utero virilization secondary to a maternal Krukenberg tumor: case report and review of literature". Journal of Pediatric Endocrinology and Metabolism. 30 (7): 785–790. doi:10.1515/jpem-2016-0433. ISSN 2191-0251. PMID 28682787. S2CID 13760498. Archived from the original on 29 July 2021. Retrieved 29 July 2021.
- ^ Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ (August 2007). "Ovarian androgen production in postmenopausal women". The Journal of Clinical Endocrinology & Metabolism. 92 (8): 3040–3. doi:10.1210/jc.2007-0581. PMID 17519304.
- ^ a b Neraud, Barbara; Dewailly, Didier (2007), Azziz, Ricardo; Nestler, John E.; Dewailly, Didier (eds.), "Drug-Induced Hyperandrogenism", Androgen Excess Disorders in Women, Contemporary Endocrinology, Totowa, NJ: Humana Press, pp. 121–127, doi:10.1007/978-1-59745-179-6_10, ISBN 978-1-58829-663-4
- ^ Neraud B, Dewailly D (8 November 2007). Azziz R, Nestler JE, Dewailly D (eds.). Contemporary Endocrinology: Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders (Second ed.). Totowa, NJ: Humana Press Inc. ISBN 978-1-59745-179-6.
- ^ Zhang, Lin; Li, Hua; Li, Shaoping; Zou, Xiaoyi (2016). "Reproductive and metabolic abnormalities in women taking valproate for bipolar disorder: a meta-analysis". European Journal of Obstetrics, Gynecology, and Reproductive Biology. 202: 26–31. doi:10.1016/j.ejogrb.2016.04.038. ISSN 1872-7654. PMID 27160812. Archived from the original on 29 July 2021. Retrieved 29 July 2021.
- ^ Spinedi, Eduardo; Cardinali, Daniel P. (2018). "The Polycystic Ovary Syndrome and the Metabolic Syndrome: A Possible Chronobiotic-Cytoprotective Adjuvant Therapy". International Journal of Endocrinology. 2018: 1349868. doi:10.1155/2018/1349868. ISSN 1687-8337. PMC 6083563. PMID 30147722.
- ^ Puttabyatappa M, Cardoso RC, Padmanabhan V (November 2016). "Effect of maternal PCOS and PCOS-like phenotype on the offspring's health". Molecular and Cellular Endocrinology. 435: 29–39. doi:10.1016/j.mce.2015.11.030. PMC 4884168. PMID 26639019.
- ^ Duan, Changling; Pei, Tianjiao; Li, Yujing; Cao, Qi; Chen, Hanxiao; Fu, Jing (2020). "Androgen levels in the fetal cord blood of children born to women with polycystic ovary syndrome: a meta-analysis". Reproductive Biology and Endocrinology. 18 (1): 81. doi:10.1186/s12958-020-00634-8. ISSN 1477-7827. PMC 7418394. PMID 32782029.
- ^ a b c Yildiz, Bulent O. (2006). "Diagnosis of hyperandrogenism: clinical criteria". Best Practice & Research Clinical Endocrinology & Metabolism. 20 (2): 167–176. doi:10.1016/j.beem.2006.02.004. ISSN 1521-690X. PMID 16772149. Archived from the original on 26 July 2021. Retrieved 29 July 2021.
- ^ a b c Apter D (October 1998). "How possible is the prevention of polycystic ovary syndrome development in adolescent patients with early onset of hyperandrogenism". Journal of Endocrinological Investigation. 21 (9): 613–7. doi:10.1007/bf03350786. PMID 9856415. S2CID 24263988.
- ^ Nader S (July 2013). "Hyperandrogenism during puberty in the development of polycystic ovary syndrome". Fertility and Sterility (Review). 100 (1): 39–42. doi:10.1016/j.fertnstert.2013.03.013. PMID 23642453.
- ^ Escobar-Morreale, H.F.; Carmina, E.; Dewailly, D.; Gambineri, A.; Kelestimur, F.; Moghetti, P.; Pugeat, M.; Qiao, J.; Wijeyaratne, C.N.; Witchel, S.F.; Norman, R.J. (2012). "Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society". Human Reproduction Update. 18 (2): 146–170. doi:10.1093/humupd/dmr042. ISSN 1460-2369. PMID 22064667. Archived from the original on 24 February 2024. Retrieved 27 July 2021.
- ^ a b Escobar-Morreale, Héctor F. (2018). "Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment". Nature Reviews Endocrinology. 14 (5): 270–284. doi:10.1038/nrendo.2018.24. ISSN 1759-5029. PMID 29569621. S2CID 4698059. Archived from the original on 29 July 2021. Retrieved 29 July 2021.
- ^ Hughes IA (November 1988). "Management of congenital adrenal hyperplasia". Archives of Disease in Childhood. 63 (11): 1399–404. doi:10.1136/adc.63.11.1399. PMC 1779155. PMID 3060026.
- ^ Merke DP, Bornstein SR (2005). "Congenital adrenal hyperplasia". Lancet. 365 (9477): 2125–36. doi:10.1016/S0140-6736(05)66736-0. PMID 15964450. S2CID 40860427.
- ^ a b Burkman RT (January 1995). "The role of oral contraceptives in the treatment of hyperandrogenic disorders". The American Journal of Medicine. 98 (1A): 130S–136S. doi:10.1016/s0002-9343(99)80071-0. PMID 7825633.
- ^ Mastorakos G, Koliopoulos C, Creatsas G (May 2002). "Androgen and lipid profiles in adolescents with polycystic ovary syndrome who were treated with two forms of combined oral contraceptives". Fertility and Sterility. 77 (5): 919–27. doi:10.1016/s0015-0282(02)02993-x. PMID 12009344.
- ^ Sinclair R, Wewerinke M, Jolley D (March 2005). "Treatment of female pattern hair loss with oral antiandrogens". The British Journal of Dermatology. 152 (3): 466–73. doi:10.1111/j.1365-2133.2005.06218.x. PMID 15787815. S2CID 26089277.
- ^ Menichini, Daniela; Facchinetti, Fabio (2020). "Effects of vitamin D supplementation in women with polycystic ovary syndrome: a review". Gynecological Endocrinology. 36 (1): 1–5. doi:10.1080/09513590.2019.1625881. ISSN 1473-0766. PMID 31187648. S2CID 186205997. Archived from the original on 29 July 2021. Retrieved 29 July 2021.
- ^ Jin, Peipei; Xie, Yongyong (2018). "Treatment strategies for women with polycystic ovary syndrome". Gynecological Endocrinology. 34 (4): 272–277. doi:10.1080/09513590.2017.1395841. ISSN 1473-0766. PMID 29084464. S2CID 4443092. Archived from the original on 30 July 2021. Retrieved 30 July 2021.
- ^ Opiyo, Newton (2019). "In women with polycystic ovary syndrome, how do lifestyle changes affect outcomes?". Cochrane Clinical Answers. doi:10.1002/cca.2649. ISSN 2050-4217. S2CID 241591297. Archived from the original on 24 February 2024. Retrieved 29 July 2021.
- ^ Escobar-Morreale, Hector F.; Santacruz, Elisa; Luque-Ramírez, Manuel; Botella Carretero, José I. (2017). "Prevalence of 'obesity-associated gonadal dysfunction' in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis". Human Reproduction Update. 23 (4): 390–408. doi:10.1093/humupd/dmx012. ISSN 1355-4786. PMID 28486593. Archived from the original on 16 June 2022. Retrieved 29 July 2021.
- ^ a b Naderpoor, Negar; Shorakae, Soulmaz; de Courten, Barbora; Misso, Marie L.; Moran, Lisa J; Teede, Helena J. (2015). "Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis". Human Reproduction Update. 21 (5): 560–574. doi:10.1093/humupd/dmv025. ISSN 1355-4786. PMID 26060208.
- ^ Edited by Adam Balen Stephen Franks Roy Homburg and Sean Kehoe Current Management of Polycystic Ovary Syndrome, edited by Adam Balen, et al., Royal College of Obstetricians and Gynaecologists, 2010. ProQuest Ebook Central (p.179)
- ^ Bermon, Stéphane; Vilain, Eric; Fénichel, Patrick; Ritzén, Martin (2015). "Women With Hyperandrogenism in Elite Sports: Scientific and Ethical Rationales for Regulating". The Journal of Clinical Endocrinology & Metabolism. 100 (3): 828–830. doi:10.1210/jc.2014-3603. ISSN 0021-972X. PMID 25587809.
- ^ "IAAF publishes briefing notes and Q&A on Female Eligibility Regulations". World Athletics. Archived from the original on 2 October 2020. Retrieved 20 September 2020.
- ^ Krishna UR, Sheriar NK (1 January 2000). "9. Hyperandrogenism in Adolescence". Adolescent Gynecology (pb). Orient Blackswan. p. 119. ISBN 978-8-12-501793-6.
- ^ Wang SQ. "Androgen Excess and PCOS Society". www.ae-society.org. Archived from the original on 16 October 2016. Retrieved 10 November 2016.
- ^ "Intersex Variations Glossary" (PDF). interactadvocates.org. 2022. Archived from the original (PDF) on 26 October 2022.
