Tetrachloro-1,2-difluoroethane
 | |
| Names | |
|---|---|
| IUPAC name
1,1,2,2-tetrachloro-1,2-difluoroethane
| |
| Other names
1,1,2,2-Tetrachloro-1,2-difluoroethane; 1,2-Difluorotetrachloroethane; Freon 112; 1,2-Difluoro-1,1,2,2-tetrachloroethane; ; sym-Tetrachlorodifluoroethane; R 112; CFC-112; 1,1,2,2-Tetrachlorodifluoroethane;
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.851 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1078 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2Cl4F2 | |
| Molar mass | 203.82 g·mol−1 |
| Appearance | clear liquid or white solid |
| Density | 1.634 g/mL |
| Melting point | 23.8 °C (74.8 °F; 296.9 K)[1] |
| Boiling point | 92.8 °C (199.0 °F; 365.9 K)[1] |
| 0.012% | |
Refractive index (nD)
|
1.4130 |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H320 | |
| P264, P264+P265, P280, P302+P352, P305+P351+P338, P321, P332+P317, P337+P317, P362+P364 | |
| Related compounds | |
Related compounds
|
CFC-112a |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
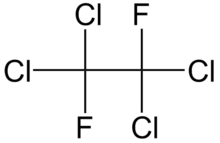
Tetrachloro-1,2-difluoroethane is a chlorofluorocarbon known as Freon 112, CFC-112 or R-112. It has a symmetrical structure CCl2FCCl2F and so can be called symmetrical tetrachlorodifluoroethane. "Symmetrical" may also be abbreviated to "s-" or "sym-". In contrast an asymmetrical isomer has formula CCl3CClF2.
Production
[edit]CFC-112 can be made in a reaction with hydrogen fluoride with hexachloroethane or tetrachloroethane with extra chlorine. This reaction occurs with an aluminium fluoride catalyst with some extra iron, nickel and chromium at 400°C. With the extra metal in the catalyst yield of the isomer can be 98% compared with the unsymmetrical isomer.[2]
Mixed with perfluorooctane, it is a solvent for polydimethylsiloxane.[3]
CFC-112 can be prepared as a mixture with other hydrochlorofluorocarbons from trichloroethylene and anhydrous hydrogen fluoride when electric current is passed through.[4]
When CFC-11 is packaged with alcohol in a metal container, a free radical reaction can result in production of CFC-112.[5]
Properties
[edit]Critical properties are critical temperature 278°C, critical pressure 4.83 MPa at a density of 0.754 g/cc.[1]
Tetrachloro-1,2-difluoroethane is not flammable.[5]
Tetrachloro-1,2-difluoroethane, like other chlorofluorocarbon compounds reacts violently with sodium, potassium or barium.[5]
Tetrachloro-1,2-difluoroethane is not very toxic, and the lethal dose is estimated at 25 g/kg. It is not carcinogenic.[5]
Use
[edit]Tetrachlorodifluoroethane (mixture of isomers) has been used as a veterinary medicine to treat parasites such as liver fluke in sheep (Fasciola hepatica).[6]
MIL-C-8638 is a military specification for a cleaning solvent that contained tetrachlorodifluoroethane, trichlorotrifluoroethane, and isopropyl alcohol. It was used to clean aircraft oxygen systems.[7]
Tetrachlorodifluoroethane can be used as an intermediate in the manufacture of tetrachloroethylene.[8]
Atmosphere
[edit]In the atmosphere of Earth, anthropogenic tetrachloro-1,2-difluoroethane has been found to occur. Between 2017 and 2020 levels, were about 0.52 parts per trillion (ppt). Levels rose from the 1970s till about 1995.[9]
References
[edit]- ^ a b c Bruno, Thomas J. (1990). Spectroscopic Library for Alternative Refrigerant Analysis. U.S. Department of Commerce, National Institute of Standards and Technology. p. 5.
- ^ Vecchio, M; Groppelli, G; Tatlow, J.C. (July 1974). "Studies on a vapour-phase process for the manufacture of chlorofluoroethanes". Journal of Fluorine Chemistry. 4 (2): 117–139. doi:10.1016/S0022-1139(00)82507-5.
- ^ Crescenzi, V.; Flory, P. J. (January 1964). "Configuration of the Poly-(dimethylsiloxane) Chain. II. Unperturbed Dimensions and Specific Solvent Effects". Journal of the American Chemical Society. 86 (2): 141–146. doi:10.1021/ja01056a006.
- ^ Polisena, C.; Liu, C. C.; Savinell, R. F. (1 December 1982). "Experimental Study of Electrochemical Fluorination of Trichloroethylene". Journal of the Electrochemical Society. 129 (12): 2720–2724. Bibcode:1982JElS..129.2720P. doi:10.1149/1.2123655.
- ^ a b c d Fully halogenated chlorofluorocarbons. Geneva: World Health Organization. 1990. p. 15. hdl:10665/39345. ISBN 9241571136.
- ^ McKellar, Quintin A.; Kinabo, Ludovick D. B. (1 July 1991). "The pharmacology of flukicidal drugs". British Veterinary Journal. 147 (4): 306–321. doi:10.1016/0007-1935(91)90003-6. PMID 1913127.
- ^ Poliakoff, M. Z (1973). "Solvent Cleaners - Where and how to Use Them". Cleaning Stainless Steel. ASTM International. p. 37.
- ^ Gottlieb, Robert (22 April 2013). Reducing Toxics: A New Approach To Policy And Industrial Decisionmaking. Island Press. p. 243. ISBN 978-1-61091-102-3.
- ^ Dunse, Bronwyn L.; Derek, Nada; Fraser, Paul J.; Krummel, Paul B. (June 2021). "Australian and Global Emissions of Ozone Depleting Substances" (PDF). CSIRO.
