Influenza vaccine
 A flu shot being given to a US Navy crew member | |
| Vaccine description | |
|---|---|
| Target | Influenza virus |
| Vaccine type | inactivated, attenuated, recombinant |
| Clinical data | |
| Trade names | Afluria, Fluarix, Fluzone, others |
| AHFS/Drugs.com | Inactivated: Monograph Intranasal: Monograph Recombinant: Monograph |
| Pregnancy category | |
| Routes of administration | Intramuscular, intranasal, intradermal |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| KEGG | |
Influenza vaccines, colloquially known as flu shots[28] or the flu jab,[29] are vaccines that protect against infection by influenza viruses.[30][31] New versions of the vaccines are developed twice a year, as the influenza virus rapidly changes.[30] While their effectiveness varies from year to year, most provide modest to high protection against influenza.[30][32] Vaccination against influenza began in the 1930s, with large-scale availability in the United States beginning in 1945.[33][34]
Both the World Health Organization and the US Centers for Disease Control and Prevention (CDC) recommend yearly vaccination for nearly all people over the age of six months, especially those at high risk,[30][35][36][37] and the influenza vaccine is on the World Health Organization's List of Essential Medicines.[38] The European Centre for Disease Prevention and Control (ECDC) also recommends yearly vaccination of high-risk groups,[39] particularly pregnant women, the elderly, children between six months and five years, and those with certain health problems.[30][37]
The vaccines are generally safe, including for people who have severe egg allergies.[40] A common side effect is soreness near the site of injection. Fever occurs in five to ten percent of children vaccinated, and temporary muscle pains or feelings of tiredness may occur. In certain years, the vaccine was linked to an increase in Guillain–Barré syndrome among older people at a rate of about one case per million doses.[30] Influenza vaccines are not recommended in those who have had a severe allergy to previous versions of the vaccine itself.[30][40] The vaccine comes in inactive and weakened viral forms. The live, weakened vaccine is generally not recommended in pregnant women, children less than two years old, adults older than 50, or people with a weakened immune system.[30] Depending on the type it can be injected into a muscle (intramuscular), sprayed into the nose (intranasal), or injected into the middle layer of the skin (intradermal).[30] The intradermal vaccine was not available during the 2018–2019 and 2019–2020 influenza seasons.[41][42][43]
History
[edit]Vaccines are used in both humans and non-humans. The human vaccine is meant unless specifically identified as a veterinary, poultry, or livestock vaccine.
Origins and development
[edit]During the worldwide Spanish flu pandemic of 1918, "Pharmacists tried everything they knew, everything they had ever heard of, from the ancient art of bleeding patients, to administering oxygen, to developing new vaccines and serums (chiefly against what we call Hemophilus influenzae – a name derived from the fact that it was originally considered the etiological agent – and several types of pneumococci). Only one therapeutic measure, transfusing blood from recovered patients to new victims, showed any hint of success."[44]
In 1931, viral growth in embryonated hens' eggs was reported by Ernest William Goodpasture and colleagues at Vanderbilt University. The work was extended to the growth of influenza virus by several workers, including Thomas Francis, Jonas Salk, Wilson Smith, and Macfarlane Burnet, leading to the first experimental influenza vaccines.[45] In the 1940s, the US military developed the first approved inactivated vaccines for influenza, which were used during World War II.[46] Hens' eggs continued to be used to produce virus used in influenza vaccines, but manufacturers made improvements in the purity of the virus by developing improved processes to remove egg proteins and to reduce systemic reactivity of the vaccine.[47] In 2012, the US Food and Drug Administration (FDA) approved influenza vaccines made by growing virus in cell cultures[48][49][50] and influenza vaccines made from recombinant proteins[51] have been approved, with plant-based influenza vaccines being tested[when?] in clinical trials.[52]
Acceptance
[edit]The egg-based technology for producing influenza vaccine was created in the 1950s.[53] In the US swine flu scare of 1976, President Gerald Ford was confronted with a potential swine flu pandemic. The vaccination program was rushed, yet plagued by delays and public relations problems. Meanwhile, maximum military containment efforts succeeded unexpectedly in confining the new strain to the single army base where it had originated. On that base, several soldiers fell severely ill, but only one died. The program was canceled after about 24% of the population had received vaccinations. An excess in deaths of 25 over normal annual levels as well as 400 excess hospitalizations, both from Guillain–Barré syndrome, were estimated to have occurred from the vaccination program itself, demonstrating that the vaccine itself is not free of risks.[54] In the end, however, even the maligned 1976 vaccine may have saved lives. A 2010 study found a significantly enhanced immune response against the 2009 pandemic H1N1 in study participants who had received vaccination against the swine flu in 1976.[55] The 2009 H1N1 "swine flu" outbreak resulted in the rapid approval of pandemic influenza vaccines.[56] Pandemrix was quickly modified to target the circulating strain and by late 2010, 70 million people had received a dose.[57] Eight years later, the BMJ gained access to early vaccine pharmacovigilance reports compiled by GSK (GlaxoSmithKline) during the pandemic, which the BMJ reported indicated death was 5.39 fold more likely with Pandemrix vs the other pandemic vaccines.[56][57] However, more thorough and robust latter analyses did not establish any increase of fatalities or most other serious adverse effects, with a possible rare exception for narcolepsy.[58]
Quadrivalent vaccines
[edit]
A quadrivalent flu vaccine administered by nasal mist was approved by the FDA in March 2012.[59][60] Fluarix Quadrivalent was approved by the FDA in December 2012.[61]
In 2014, the Canadian National Advisory Committee on Immunization (NACI) published a review of quadrivalent influenza vaccines.[62]
Starting with the 2018–2019 influenza season most of the regular-dose egg-based flu shots and all the recombinant and cell-grown flu vaccines in the United States are quadrivalent.[63] In the 2019–2020 influenza season all regular-dose flu shots and all recombinant influenza vaccine in the United States are quadrivalent.[64]
In November 2019, the FDA approved Fluzone High-Dose Quadrivalent for use in the United States starting with the 2020–2021 influenza season.[65][66]
In February 2020, the FDA approved Fluad Quadrivalent for use in the United States.[67][68] In July 2020, the FDA approved both Fluad and Fluad Quadrivalent for use in the United States for the 2020–2021 influenza season.[67][69]
The B/Yamagata lineage of influenza B, one of the four lineages targeted by quadrivalent vaccines, might have become extinct in 2020/2021 due to COVID-19 pandemic measures,[70] and there have been no naturally occurring cases confirmed since March 2020.[71][72] In 2023, the World Health Organization concluded that protection against the Yamagata lineage was no longer necessary in the seasonal flu vaccine, so future vaccines are recommended to be trivalent instead of quadrivalent.[71][72] For the 2024–2025 Northern Hemisphere influenza season, the FDA recommends removing B/Yamagata from all influenza vaccines.[73]
Medical uses
[edit]The influenza vaccine is indicated for active immunization for the prevention of influenza disease caused by influenza virus subtypes A and type B contained in the vaccine.[74][75][76]
The US Centers for Disease Control and Prevention (CDC) recommends the flu vaccine as the best way to protect people against the flu and prevent its spread.[77] The flu vaccine can also reduce the severity of the flu if a person contracts a strain that the vaccine did not contain.[77] It takes about two weeks following vaccination for protective antibodies to form.[77][78]
A 2012 meta-analysis found that flu vaccination was effective 67 percent of the time; the populations that benefited the most were HIV-positive adults aged 18 to 55 (76 percent), healthy adults aged 18 to 46 (approximately 70 percent), and healthy children aged six months to 24 months (66 percent).[79] The influenza vaccine also appears to protect against myocardial infarction with a benefit of 15–45%.[80]
Effectiveness
[edit]Graphs are unavailable due to technical issues. Updates on reimplementing the Graph extension, which will be known as the Chart extension, can be found on Phabricator and on MediaWiki.org. |
A vaccine is assessed by its efficacy – the extent to which it reduces the risk of disease under controlled conditions – and its effectiveness – the observed reduction in risk after the vaccine is put into use.[87] In the case of influenza, effectiveness is expected to be lower than the efficacy because it is measured using the rates of influenza-like illness, which is not always caused by influenza.[88] Studies on the effectiveness of flu vaccines in the real world are difficult; vaccines may be imperfectly matched, virus prevalence varies widely between years, and influenza is often confused with other influenza-like illnesses.[89] However, in most years (16 of the 19 years before 2007), the flu vaccine strains have been a good match for the circulating strains,[90] and even a mismatched vaccine can often provide cross-protection.[77] The virus rapidly changes due to antigenic drift, a slight mutation in the virus that causes a new strain to arise.[91]
The effectiveness of seasonal flu vaccines varies significantly, with an estimated average efficacy of 50–60% against symptomatic disease,[92] depending on vaccine strain, age, prior immunity, and immune function, so vaccinated people can still contract influenza.[93] The effectiveness of flu vaccines is considered to be suboptimal, particularly among the elderly,[94] but vaccination is still beneficial in reducing the mortality rate and hospitalization rate due to influenza as well as duration of hospitalization.[93][95] Vaccination of school-age children has shown to provide indirect protection for other age groups. LAIVs are recommended for children based on superior efficacy, especially for children under 6, and greater immunity against non-vaccine strains when compared to inactivated vaccines.[96][97]
From 2012 to 2015 in New Zealand, vaccine effectiveness against admission to an intensive care unit was 82%.[98] Effectiveness against hospitalized influenza illness in the 2019–2020 United States flu season was 41% overall and 54% in people aged 65 years or older.[99] One review found 31% effectiveness against death among adults.[100][101]
Repeated annual influenza vaccination generally offers consistent year-on-year protection against influenza.[101] There is, however, suggestive evidence that repeated vaccinations may cause a reduction in vaccine effectiveness for certain influenza subtypes; this has no relevance to recommendations for yearly vaccinations but might influence future vaccination policy.[102][103] As of 2019[update], the CDC recommends a yearly vaccine as most studies demonstrate overall effectiveness of annual influenza vaccination.[101]
There is not enough evidence to establish significant differences in the effectiveness of different influenza vaccine types,[104] but there are high-dose or adjuvanted products that induce a stronger immune response in the elderly.[105]
According to a 2016 study by faculty at the University of New South Wales, getting a flu shot was as effective or better at preventing a heart attack than even quitting smoking.[106]
A 2024 CDC study found that the 2024 flu vaccine reduced the risk of hospitalization from the flu by 35% in the Southern Hemisphere.[107] The research, conducted across five countries—Argentina, Brazil, Chile, Paraguay, and Uruguay—showed the vaccine was less effective than the one used in the previous season.[108]
Children
[edit]In April 2002, the Advisory Committee on Immunization Practices (ACIP) encouraged that children 6 to 23 months of age be vaccinated annually against influenza.[109] In 2010, ACIP recommended annual influenza vaccination for those 6 months of age and older.[109] The CDC recommends that everyone except infants under the age of six months should receive the seasonal influenza vaccine.[35] Vaccination campaigns usually focus special attention on people who are at high risk of serious complications if they catch the flu, such as pregnant women, children under 59 months, the elderly, and people with chronic illnesses or weakened immune systems, as well as those to whom they are exposed, such as health care workers.[35][110]
As the death rate is also high among infants who catch influenza, the CDC and the WHO recommend that household contacts and caregivers of infants be vaccinated to reduce the risk of passing an influenza infection to the infant.[110][111]
In children, the vaccine appears to decrease the risk of influenza and possibly influenza-like illness.[112] In children under the age of two data are limited.[112] During the 2017–18 flu season, the CDC director indicated that 85 percent of the children who died "likely will not have been vaccinated".[113]
In the United States, as of January 2019[update], the CDC recommend that children aged six through 35 months may receive either 0.25 milliliters or 0.5 milliliters per dose of Fluzone Quadrivalent.[64][114] There is no preference for one or the other dose volume of Fluzone Quadrivalent for that age group.[64] All persons 36 months of age and older should receive 0.5 milliliters per dose of Fluzone Quadrivalent.[64] As of October 2018[update], Afluria Quadrivalent is licensed for children six months of age and older in the United States.[64][115] Children six months through 35 months of age should receive 0.25 milliliters for each dose of Afluria Quadrivalent.[64] All persons 36 months of age and older should receive 0.5 milliliters per dose of Afluria Quadrivalent.[64] As of February 2018[update], Afluria Tetra is licensed for adults and children five years of age and older in Canada.[116]
In 2014, the Canadian National Advisory Committee on Immunization (NACI) published a review of influenza vaccination in healthy 5–18-year-olds,[117] and in 2015, published a review of the use of pediatric Fluad in children 6–72 months of age.[118] In one study, conducted in a tertiary referral center, the rate of influenza vaccination in children was only 31%. Higher rates were found among immuno-suppressed pediatric patients (46%) and in patients with inflammatory bowel disease (50%).[119]
Adults
[edit]
In unvaccinated adults, 16% get symptoms similar to the flu, while about 10% of vaccinated adults do.[88] Vaccination decreased confirmed cases of influenza from about 2.4% to 1.1%.[88] No effect on hospitalization was found.[88]
In working adults, a review by the Cochrane Collaboration found that vaccination resulted in a modest decrease in both influenza symptoms and working days lost, without affecting transmission or influenza-related complications.[88] In healthy working adults, influenza vaccines can provide moderate protection against virologically confirmed influenza, though such protection is greatly reduced or absent in some seasons.[120]
In health care workers, a 2006 review found a net benefit.[121] Of the eighteen studies in this review, only two also assessed the relationship of patient mortality relative to staff influenza vaccine uptake; both found that higher rates of healthcare worker vaccination correlated with reduced patient deaths.[121] A 2014 review found benefits to patients when health care workers were immunized, as supported by moderate evidence[122] based in part on the observed reduction in all-cause deaths in patients whose health care workers were given immunization compared with comparison patients where the workers were not offered the vaccine.[123]
Elderly
[edit]Evidence for an effect in adults over 65 is unclear.[124] Systematic reviews examining both randomized controlled and case–control studies found a lack of high-quality evidence.[120][125] Reviews of case-control studies found effects against laboratory-confirmed influenza, pneumonia, and death among the community-dwelling elderly.[126][127]
The group most vulnerable to non-pandemic flu, the elderly, benefits least from the vaccine. There are multiple reasons behind this steep decline in vaccine efficacy, the most common of which are the declining immunological function and frailty associated with advanced age.[128] In a non-pandemic year, a person in the United States aged 50–64 is nearly ten times more likely to die an influenza-associated death than a younger person, and a person over 65 is more than ten times more likely to die an influenza-associated death than the 50–64 age group.[129]
There is a high-dose flu vaccine specifically formulated to provide a stronger immune response.[130] Available evidence indicates that vaccinating the elderly with the high-dose vaccine leads to a stronger immune response against influenza than the regular-dose vaccine.[131][132][133]
A flu vaccine containing an adjuvant was approved by the US Food and Drug Administration (FDA) in November 2015, for use by adults aged 65 years of age and older. The vaccine is marketed as Fluad in the US and was first available in the 2016–2017 flu season. The vaccine contains the MF59C.1 adjuvant[134] which is an oil-in-water emulsion of squalene oil. It is the first adjuvanted seasonal flu vaccine marketed in the United States.[135][136][137] It is not clear if there is a significant benefit for the elderly to use a flu vaccine containing the MF59C.1 adjuvant.[138][139][140] Per Advisory Committee on Immunization Practices guidelines, Fluad can be used as an alternative to other influenza vaccines approved for people 65 years and older.[136]
Vaccinating healthcare workers who work with elderly people is recommended in many countries, with the goal of reducing influenza outbreaks in this vulnerable population.[141][142][143] While there is no conclusive evidence from randomized clinical trials that vaccinating health care workers helps protect elderly people from influenza, there is tentative evidence of benefit.[144]
Fluad Quad was approved for use in Australia in September 2019,[145] Fluad Quadrivalent was approved for use in the United States in February 2020,[67] and Fluad Tetra was authorized for use in the European Union in May 2020.[146][147]
Pregnancy
[edit]As well as protecting mother and child from the effects of an influenza infection, the immunization of pregnant women tends to increase their chances of experiencing a successful full-term pregnancy.[148]
The trivalent inactivated influenza vaccine is protective in pregnant women infected with HIV.[149]
Safety
[edit]Side effects
[edit]Common side effects of vaccination include local injection-site reactions and cold-like symptoms. Fever, malaise, and myalgia are less common. Flu vaccines are contraindicated for people who have experienced a severe allergic reaction in response to a flu vaccine or to any component of the vaccine. LAIVs are not given to children or adolescents with severe immunodeficiency or to those who are using salicylate treatments because of the risk of developing Reye syndrome.[96] LAIVs are also not recommended for children under the age of 2,[97] pregnant women, and adults with immunosuppression. Inactivated flu vaccines cannot cause influenza and are regarded as safe during pregnancy.[96]
While side effects of the flu vaccine may occur, they are usually minor, including soreness, redness, swelling around the point of injection, headache, fever, nausea, or fatigue.[150] Side effects of a nasal spray vaccine may include runny nose, wheezing, sore throat, cough, or vomiting.[151]
In some people, a flu vaccine may cause serious side effects, including an allergic reaction, but this is rare. Furthermore, the common side effects and risks are mild and temporary when compared to the risks and severe health effects of the annual influenza epidemic.[77]
Contrary to a common misconception, flu shots cannot cause people to get the flu.[152][153]
Guillain–Barré syndrome
[edit]Although Guillain–Barré syndrome had been feared as a complication of vaccination, the CDC states that most studies on modern influenza vaccines have seen no link with Guillain–Barré.[154][155] Infection with influenza virus itself increases both the risk of death (up to one in ten thousand) and the risk of developing Guillain–Barré syndrome to a far higher level than the highest level of suspected vaccine involvement (approximately ten times higher by 2009 estimates).[156][157]
Although one review gives an incidence of about one case of Guillain–Barré per million vaccinations,[158] a large study in China, covering close to a hundred million doses of vaccine against the 2009 H1N1 "swine" flu found only eleven cases of Guillain–Barré syndrome, (0.1 per million doses) total incidence in persons vaccinated, actually lower than the normal rate of the disease in China, and no other notable side effects.[157][159]
Egg allergy
[edit]
Although most influenza vaccines are produced using egg-based techniques, influenza vaccines are nonetheless still recommended as safe for people with egg allergies, even if severe,[40] as no increased risk of allergic reaction to the egg-based vaccines has been shown for people with egg allergies.[160] Studies examining the safety of influenza vaccines in people with severe egg allergies found that anaphylaxis was very rare, occurring in 1.3 cases per million doses given.[40]
Monitoring for symptoms from vaccination is recommended in those with more severe symptoms.[161] A study of nearly 800 children with egg allergy, including over 250 with previous anaphylactic reactions, had zero systemic allergic reactions when given the live attenuated flu vaccine.[162][163]
Vaccines produced using other technologies, notably recombinant vaccines and those based on cell culture rather than egg protein, started to become available in 2012 in the US,[164] and later in Europe[165] and Australia.[160]
Other
[edit]Several studies have identified an increased incidence of narcolepsy among recipients of the pandemic H1N1 influenza AS03-adjuvanted vaccine;[166] efforts to identify a mechanism for this suggest that narcolepsy is autoimmune, and that the AS03-adjuvanted H1N1 vaccine may mimic hypocretin, serving as a trigger.[167]
Some injection-based flu vaccines intended for adults in the United States contain thiomersal (also known as thimerosal), a mercury-based preservative.[168][169] Despite some controversy in the media,[170] the World Health Organization's Global Advisory Committee on Vaccine Safety has concluded that there is no evidence of toxicity from thiomersal in vaccines and no reason on grounds of safety to change to more-expensive single-dose administration.[171]
Exercising before the influenza vaccine is not thought to be harmful but there is no evidence of a beneficial effect either.[172]
Types
[edit]
Seasonal flu vaccines are available either as:[citation needed]
- a trivalent or quadrivalent injection, which contains the inactivated form of the virus. This is usually an intramuscular injection, though subcutaneous and intradermal routes can also be protective.[173]
- a nasal spray of live attenuated influenza vaccine, which contains the live but attenuated (weakened) form of the virus.
Injected vaccines induce protection based on an immune response to the antigens present on the inactivated virus, while the nasal spray works by establishing short-term infection in the nasal passages.[174]
Annual reformulation
[edit]Each year, three influenza strains are chosen for inclusion in the forthcoming year's seasonal flu vaccination by the Global Influenza Surveillance and Response System of the World Health Organization (WHO).[175] The recommendation for trivalent vaccine comprises two strains of Influenza A (one each of A/H1N1 and A/H3N2), and one strain of influenza B (B/Victoria), together representing strains thought most likely to cause significant human suffering in the coming season. Starting in 2012, WHO has also recommended a second influenza B strain (B/Yamagata) for use in quadrivalent vaccines; this was discontinued in 2024.[176]
- "The WHO Global Influenza Surveillance Network was established in 1952 (renamed "Global Influenza Surveillance and Response System" in 2011).[177] The network comprises four WHO Collaborating Centres (WHO CCs) and 112 institutions in 83 countries, which are recognized by WHO as WHO National Influenza Centres (NICs). These NICs collect specimens in their country and perform primary virus isolation and preliminary antigenic characterization. They ship newly isolated strains to WHO CCs for high-level antigenic and genetic analysis, the result of which forms the basis for WHO recommendations on the composition of influenza vaccine for the Northern and Southern Hemisphere each year."[178]
Formal WHO recommendations were first issued in 1973. Beginning in 1999 there have been two recommendations per year: one for the northern hemisphere and the other for the southern hemisphere.[179]
Due to the widespread use of non-pharmaceutical interventions at the beginning of the COVID-19 pandemic, the B/Yamagata influenza lineage has not been isolated since March 2020 and may have been eradicated. Starting with the 2024 Southern Hemisphere influenza season, the WHO and other regulatory bodies have removed B/Yamagata from influenza vaccine recommendations.[176][73][180]
Recommendations
[edit]Various public health organizations, including the World Health Organization (WHO), recommend that yearly influenza vaccination be routinely offered, particularly to people at risk of complications of influenza and those individuals who live with or care for high-risk individuals, including:
- people aged 50 years of age or older[37]
- people with chronic lung diseases, including asthma[37]
- people with chronic heart diseases[37]
- people with chronic liver diseases[37]
- people with chronic kidney diseases[37]
- people who have had their spleen removed or whose spleen is not working properly[medical citation needed]
- people who are immunocompromised[37]
- residents of nursing homes and other long-term care facilities[37]
- health care workers (both to prevent sickness and to prevent spread to their patients)[181][182]
- women who are or will be pregnant during the influenza season[37]
- children and adolescents (aged 6 months through 18 years) who are receiving aspirin- or salicylate-containing medications and who might be at risk for experiencing Reye syndrome after influenza virus infection[37]
- American Indians/Alaska Natives[37]
- people who are extremely obese (body mass index ≥40 for adults)[37]
The flu vaccine is contraindicated for those under six months of age and those with severe, life-threatening allergies to flu vaccine or any ingredient in the vaccine.[35][183][40]
World Health Organization
[edit]As of 2016[update], the World Health Organization (WHO) recommends seasonal influenza vaccination for:[184][185][186][187][188]
First priority:
- Pregnant women
Second priority (in no particular order):
- Children aged 6–59 months
- Elderly
- Individuals with specific chronic medical conditions
- Health-care workers
Canada
[edit]The National Advisory Committee on Immunization (NACI), the group that advises the Public Health Agency of Canada, recommends that everyone over six months of age be encouraged to receive annual influenza vaccination and that children between the age of six months and 24 months, and their household contacts, should be considered a high priority for the flu vaccine.[189] Particularly:
- People at high risk of influenza-related complications or hospitalization, including people who are morbidly obese, healthy pregnant women, children aged 6–59 months, the elderly, aboriginals, and people with one of an itemized list of chronic health conditions
- People capable of transmitting influenza to those at high risk, including household contacts and healthcare workers
- People who provide essential community services
- Certain poultry workers
Live attenuated influenza vaccine (LAIV) was not available in Canada for the 2019–2020 season.[189]
European Union
[edit]The European Centre for Disease Prevention and Control (ECDC) recommends vaccinating the elderly as a priority, with a secondary priority for people with chronic medical conditions and health care workers.[190]
The influenza vaccination strategy is generally that of protecting vulnerable people, rather than limiting influenza circulation or eliminating human influenza sickness. This is in contrast with the high herd immunity strategies for other infectious diseases such as polio and measles.[191] This is also due in part to the financial and logistics burden associated with the need of an annual injection.[192]
United Kingdom
[edit]The National Health Service in the United Kingdom provides flu vaccination to:
- people who are aged 65 or over
- people who have certain long-term health conditions
- people who are pregnant
- people who live in a care home
- people who are the main carer for an older or disabled person, or receive a carer's allowance
- people who live with someone who has a weakened immune system.[193]
This vaccination is available free of charge to people in these groups. People outside these groups aged between 18 and 65 years of age can also receive private flu vaccination for a small fee from pharmacies and some private surgeries.[194]
United States
[edit]
In the United States routine influenza vaccination is recommended for all persons aged six months and over.[195][37][196] It takes up to two weeks after vaccination for sufficient antibodies to develop in the body.[196] The CDC recommends vaccination before the end of October,[37] although it considers getting a vaccine in December or even later to be still beneficial.[37][77][196] The U.S. military also requires a flu shot annually for its active and reserve servicemembers.[197]
According to the CDC, the live attenuated virus (LAIV4) (which comes in the form of nasal spray in the US) should be avoided by some groups.[37][198]
Within its blanket recommendation for general vaccination in the United States, the CDC, which began recommending the influenza vaccine to healthcare workers in 1981, emphasizes to clinicians the special urgency of vaccination for members of certain vulnerable groups, and their caregivers:
- Vaccination is especially important for people at higher risk of serious influenza complications or people who live with or care for people at higher risk for serious complications.[199] In 2009, a new high-dose formulation of the standard influenza vaccine was approved.[200] The Fluzone High Dose is specifically for people 65 and older; the difference is that it has four times the antigen dose of the standard Fluzone.[201][202][203][204]
The US government requires hospitals to report worker vaccination rates. Some US states and hundreds of US hospitals require healthcare workers to either get vaccinations or wear masks during flu season. These requirements occasionally engender union lawsuits on narrow collective bargaining grounds, but proponents note that courts have generally endorsed forced vaccination laws affecting the general population during disease outbreaks.[205]
Vaccination against influenza is especially considered important for members of high-risk groups who would be likely to have complications from influenza, for example pregnant women[37][206] and children and teenagers from six months to 18 years of age who are receiving aspirin- or salicylate-containing medications and who might be at risk for experiencing Reye syndrome after influenza virus infection;[37]
- In raising the upper age limit to 18 years, the aim is to reduce both the time children and parents lose from visits to pediatricians and missing school and the need for antibiotics for complications[207]
- An added benefit expected from the vaccination of children is a reduction in the number of influenza cases among parents and other household members, and of possible spread to the general community.[207]
The CDC indicated that live attenuated influenza vaccine (LAIV), also called the nasal spray vaccine, was not recommended for the 2016–2017 flu season in the United States.[208]
Furthermore, the CDC recommends that healthcare personnel who care for severely immunocompromised persons receive injections (TIV or QIV) rather than LAIV.[209]
Australia
[edit]The Australian Government recommends seasonal flu vaccination for everyone over the age of six months. Australia uses inactivated vaccines.[210] Until 2021, the egg-based vaccine has been the only one available (and continues to be the only free one), but from March 2021 a new cell-based vaccine is available for those who wish to pay for it, and it is expected that this one will become the standard by 2026.[160] The standard flu vaccine is free for the following people:[211]
- children aged six months to five years;
- people aged 65 years and over;
- Aboriginal and Torres Strait Islander people aged six months and over;
- pregnant women; and
- anyone over six months of age with medical conditions such as severe asthma, lung disease or heart disease, low immunity, or diabetes that can lead to complications from influenza.
Uptake
[edit]| Country | Region | % aged 65+ |
|---|---|---|
| Republic of Korea | Asia | 83 |
| Australia | Oceania | 75 |
| United Kingdom | Europe | 73 |
| United States | Americas | 68 |
| New Zealand | Oceania | 65 |
| Chile | Americas | 65 |
| Netherlands | Europe | 64 |
| Canada | Americas | 61 |
| Portugal | Europe | 61 |
| Israel | Asia | 58 |
| Ireland | Europe | 58 |
| Spain | Europe | 54 |
| Italy | Europe | 53 |
| Denmark | Europe | 52 |
| Japan | Asia | 50 |
| France | Europe | 50 |
| Sweden | Europe | 49 |
| Finland | Europe | 48 |
| Iceland | Europe | 45 |
| Luxembourg | Europe | 38 |
| Germany | Europe | 35 |
| Norway | Europe | 34 |
| Hungary | Europe | 27 |
| Czech Republic | Europe | 20 |
| Lithuania | Europe | 13 |
| Slovak Republic | Europe | 13 |
| Slovenia | Europe | 12 |
| Latvia | Europe | 8 |
| Turkey | Asia | 7 |
| Estonia | Europe | 5 |
At risk groups
[edit]Uptake of flu vaccination, both seasonally and during pandemics, is often low.[213] Systematic reviews of pandemic flu vaccination uptake have identified several personal factors that may influence uptake, including gender (higher uptake in men), ethnicity (higher in people from ethnic minorities), and having a chronic illness.[214][215] Beliefs in the safety and effectiveness of the vaccine are also important.[213]
Several measures are useful to increase rates of vaccination in those over sixty including patient reminders using leaflets and letters, postcard reminders, client outreach programs, vaccine home visits, group vaccinations, free vaccinations, physician payment, physician reminders, and encouraging physician competition.[216]
Health care workers
[edit]Frontline healthcare workers are often recommended to get seasonal and any pandemic flu vaccinations. For example, in the UK all healthcare workers involved in patient care are recommended to receive the seasonal flu vaccine, and were also recommended to be vaccinated against the H1N1/09 (later renamed A(H1N1)pdm09[note 1][217]) swine flu virus during the 2009 pandemic. However, uptake is often low.[182] During the 2009 pandemic, low uptake by healthcare workers was seen in countries including the UK,[182] Italy,[218] Greece,[219] and Hong Kong.[220]
In a 2010 survey of United States healthcare workers, 63.5% reported that they received the flu vaccine during the 2010–11 season, an increase from 61.9% reported the previous season. US Health professionals with direct patient contact had higher vaccination uptake, such as physicians and dentists (84.2%) and nurse practitioners (82.6%).[221][222][223]
The main reason to vaccinate health care workers is to prevent staff from spreading flu to their patients and to reduce staff absence at a time of high service demand, but the reasons health care workers state for their decisions to accept or decline vaccination may more often be to do with perceived personal benefits.[182]
In Victoria (Australia) public hospitals, rates of healthcare worker vaccination in 2005 ranged from 34% for non-clinical staff to 42% for laboratory staff. One of the reasons for rejecting vaccines was concern over adverse reactions; in one study, 31% of resident physicians at a teaching hospital incorrectly believed Australian vaccines could cause influenza.[224]
Manufacturing
[edit]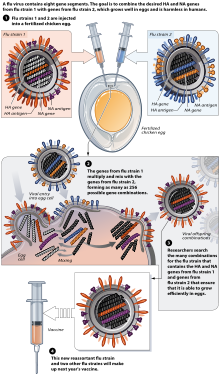
Research continues into the idea of a "universal" influenza vaccine that would not require tailoring to a particular strain, but would be effective against a broad variety of influenza viruses.[225] No vaccine candidates had been announced by November 2007,[226] but as of 2021[update], there are several universal vaccines candidates, in pre-clinical development and in clinical trials.[227][228][229][230]
In a 2007 report, the global capacity of approximately 826 million seasonal influenza vaccine doses (inactivated and live) was double the production of 413 million doses. In an aggressive scenario of producing pandemic influenza vaccines by 2013, only 2.8 billion courses could be produced in a six-month time frame. If all high- and upper-middle-income countries sought vaccines for their entire populations in a pandemic, nearly two billion courses would be required. If China pursued this goal as well, more than three billion courses would be required to serve these populations.[231] Vaccine research and development is ongoing to identify novel vaccine approaches that could produce much greater quantities of vaccine at a price that is affordable to the global population.[citation needed]
Egg-based
[edit]Most flu vaccines are grown by vaccine manufacturers in fertilized chicken eggs.[232][226] In the Northern hemisphere, the manufacturing process begins following the announcement (typically in February) of the WHO recommended strains for the winter flu season.[232][233] Three strains (representing an H1N1, an H3N2, and a B strain) of flu are selected and chicken eggs are inoculated separately. These monovalent harvests are then combined to make the trivalent vaccine.[234]
As of November 2007[update], both the conventional injection and the nasal spray are manufactured using chicken eggs. The European Union also approved Optaflu, a vaccine produced by Novartis using vats of animal cells. This technique is expected to be more scalable and avoid problems with eggs, such as allergic reactions and incompatibility with strains that affect avians like chickens.[226]
Influenza vaccines are produced in pathogen-free eggs that are eleven or twelve days old.[235] The top of the egg is disinfected by wiping it with alcohol and then the egg is candled to identify a non-veinous area in the allantoic cavity where a small hole is poked to serve as a pressure release.[236] A second hole is made at the top of the egg, where the influenza virus is injected in the allantoic cavity, past the chorioallantoic membrane. The two holes are then sealed with melted paraffin and the inoculated eggs are incubated for 48 hours at 37 degrees Celsius.[235] During the incubation time, the virus replicates and newly replicated viruses are released into the allantoic fluid[237]
After the 48-hour incubation period, the top of the egg is cracked and ten milliliters of allantoic fluid is removed, from which about fifteen micrograms of the flu vaccine can be obtained. At this point, the viruses have been weakened or killed and the viral antigen is purified and placed inside vials, syringes, or nasal sprayers.[237] Up to 3 eggs are needed to produce one dose of a trivalent vaccine, and an estimated 600 million eggs are produced each year for flu vaccine production.[238]
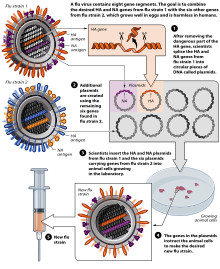
Other methods of manufacture
[edit]Methods of vaccine generation that bypass the need for eggs include the construction of influenza virus-like particles (VLP). VLP resemble viruses, but there is no need for inactivation, as they do not include viral coding elements, but merely present antigens in a similar manner to a virion. Some methods of producing VLP include cultures of Spodoptera frugiperda Sf9 insect cells and plant-based vaccine production (e.g., production in Nicotiana benthamiana). There is evidence that some VLPs elicit antibodies that recognize a broader panel of antigenically distinct viral isolates compared to other vaccines in the hemagglutination-inhibition assay (HIA).[239]
A gene-based DNA vaccine, used to prime the immune system after boosting with an inactivated H5N1 vaccine, underwent clinical trials in 2011.[240][241][242]
In November 2012, Novartis received FDA approval for the first cell-culture vaccine.[164][49][243][244] In 2013, the recombinant influenza vaccine, Flublok, was approved for use in the United States.[51][245][246][247]
On September 17, 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for Supemtek, a quadrivalent influenza vaccine (recombinant, prepared in cell culture).[248] The applicant for this medicinal product is Sanofi Pasteur.[248] Supemtek was authorized for medical use in the European Union in November 2020.[165][249]
Australia authorized its first cell-based vaccine in March 2021, based on an "eternal cell line" of a dog kidney. Because of the way it is produced, it produces better-matched vaccines (to the flu strains).[160]
Vaccine manufacturing countries
[edit]According to the WHO, as of 2019[update], countries where influenza vaccine is produced include:[250]
- Australia
- Brazil
- Canada
- China
- France
- Germany
- Hungary
- India
- Iran
- Japan
- Mexico
- Netherlands
- Nicaragua
- Russian Federation
- South Korea
- United Kingdom
- United States
- Vietnam
In addition, Kazakhstan, Serbia, and Thailand had facilities in the final stages of establishing production.[250]
Cost-effectiveness
[edit]The cost-effectiveness of seasonal influenza vaccination has been widely evaluated for different groups and in different settings.[251] In the elderly (over 65), the majority of published studies have found that vaccination is cost-saving, with the cost savings associated with influenza vaccination (e.g. prevented health care visits) outweighing the cost of vaccination.[252] In older adults (aged 50–64 years), several published studies have found that influenza vaccination is likely to be cost-effective, however, the results of these studies were often found to be dependent on key assumptions used in the economic evaluations.[253] The uncertainty in influenza cost-effectiveness models can partially be explained by the complexities involved in estimating the disease burden,[254] as well as the seasonal variability in the circulating strains and the match of the vaccine.[255][256] In healthy working adults (aged 18–49 years), a 2012 review found that vaccination was generally not cost-saving, with the suitability for funding being dependent on the willingness to pay to obtain the associated health benefits.[257] In children, the majority of studies have found that influenza vaccination was cost-effective, however many of the studies included (indirect) productivity gains, which may not be given the same weight in all settings.[258] Several studies have attempted to predict the cost-effectiveness of interventions (including pre-pandemic vaccination) to help protect against a future pandemic, however estimating the cost-effectiveness has been complicated by uncertainty as to the severity of a potential future pandemic and the efficacy of measures against it.[259]
Research
[edit]Influenza research includes molecular virology, molecular evolution, pathogenesis, host immune responses, genomics, and epidemiology. These help in developing influenza countermeasures such as vaccines, therapies, and diagnostic tools. Improved influenza countermeasures require basic research on how viruses enter cells, replicate, mutate, evolve into new strains, and induce an immune response. The Influenza Genome Sequencing Project is creating a library of influenza sequences[260] that will help researchers' understanding of what makes one strain more lethal than another, what genetic determinants most affect immunogenicity, and how the virus evolves.
A different approach uses Internet content to estimate the impact of an influenza vaccination campaign. More specifically, researchers have used data from Twitter and Microsoft's Bing search engine and proposed a statistical framework that, after a series of operations, maps this information to estimates of the influenza-like illness reduction percentage in areas where vaccinations have been performed. The method has been used to quantify the impact of two flu vaccination programmes in England (2013/14 and 2014/15), where school-age children were administered a live attenuated influenza vaccine (LAIV). Notably, the impact estimates were in accordance with estimations from Public Health England based on traditional syndromic surveillance endpoints.[261][262]
Rapid response to pandemic flu
[edit]The rapid development, production, and distribution of pandemic influenza vaccines could potentially save millions of lives during an influenza pandemic. Due to the short time frame between the identification of a pandemic strain and the need for vaccination, researchers are looking at novel technologies for vaccine production that could provide better "real-time" access and be produced more affordably, thereby increasing access for people living in low- and moderate-income countries, where an influenza pandemic may likely originate, such as live attenuated (egg-based or cell-based) technology and recombinant technologies (proteins and virus-like particles).[263] As of July 2009[update], more than seventy known clinical trials have been completed or are ongoing for pandemic influenza vaccines.[264] In September 2009, the FDA approved four vaccines against the 2009 H1N1 influenza virus (the 2009 pandemic strain), and expected the initial vaccine lots to be available within the following month.[265]
In January 2020, the US Food and Drug Administration (FDA) approved Audenz as a vaccine for the H5N1 flu virus.[266] Audenz is a vaccine indicated for active immunization for the prevention of disease caused by the influenza A virus H5N1 subtype contained in the vaccine. Audenz is approved for use in persons six months of age and older at increased risk of exposure to the influenza A virus H5N1 subtype contained in the vaccine.[267]
Zoonotic influenza vaccine Seqirus is authorized for use in the European Union.[268] It is an H5N8 vaccine that is intended to provide acquired immunity against H5 subtype influenza A viruses.[268]
Universal flu vaccines
[edit]A universal influenza vaccine that would not have to be designed and made for each flu season in each hemisphere would stabilize the supply, avoid errors in predicting the season's variants, and protect against the escape of the circulating strains by mutation.[225] Such a vaccine has been the subject of research for decades.[269]
One approach is to use broadly neutralizing antibodies that, unlike the annual seasonal vaccines used over the first decades of the 21st century that provoke the body to generate an immune response, instead provide a component of the immune response itself. The first neutralizing antibodies were identified in 1993, via experimentation.[270] It was found that the flu neutralizing antibodies bound to the stalk of the Hemagglutinin protein. Antibodies that could bind to the head of those proteins were identified. The highly conserved M2 proton channel was proposed as a potential target for broadly neutralizing antibodies.[269][271]
The challenges for researchers are to identify single antibodies that could neutralize many subtypes of the virus so that they could be useful in any season, and that target conserved domains that are resistant to antigenic drift.[269]
Another approach is to take the conserved domains identified from these projects, and to deliver groups of these antigens to provoke an immune response; various approaches with different antigens, presented in different ways (as fusion proteins, mounted on virus-like particles, on non-pathogenic viruses, as DNA, and others), are under development.[271][272][273]
Efforts have also been undertaken to develop universal vaccines that specifically activate a T-cell response, based on clinical data showing that people with a strong, early T-cell response have better outcomes when infected with influenza and because T-cells respond to conserved epitopes. The challenge for developers is that these epitopes are on internal protein domains that are only mildly immunogenic.[271]
Along with the rest of the vaccine field, people working on universal vaccines have experimented with vaccine adjuvants to improve the ability of their vaccines to create a sufficiently powerful and enduring immune response.[271][274]
Oral influenza vaccine
[edit]As of 2019, an oral flu vaccine was in clinical research.[275] The oral vaccine candidate is based on an adenovirus type 5 vector modified to remove genes needed for replication, with an added gene that expresses a small double-stranded RNA hairpin molecule as an adjuvant.[276] In 2020, a phase II human trial of the pill form of the vaccine showed that it was well tolerated and provided similar immunity to a licensed injectable vaccine.[277]
Possible Pleiotropic Effects
[edit]Recent observational studies and clinical trials suggest nonspecific effects of influenza vaccination, known as pleiotropic effects, with broader impact beyond protecting against influenza infection. A meta-analysis of 9001 randomized trial participants found that influenza vaccination was associated with a 34% lower risk of major adverse cardiovasular events when compared to placebo.[278] This risk reduction size is comparable to the cardioprotective effects seen with other guideline-recommended cardiovascular medications, including statins.[279] Protection against stroke of all etiologies has also been suggested in a large population-based retrospective cohort study of 4 million adults in Canada.[280] There may also be protective effects against the development of type 1 diabetes and cancer-related mortality, which are active areas of investigation.[281]
COVID-19
[edit]An influenza vaccine and a COVID-19 vaccine may be given safely at the same time.[78][282] Preliminary research indicates that influenza vaccination does not prevent COVID-19, but may reduce the incidence and severity of COVID-19 infection.[283]
Criticism
[edit]Tom Jefferson, who has led Cochrane Collaboration reviews of flu vaccines, has called clinical evidence concerning flu vaccines "rubbish" and has therefore declared them to be ineffective; he has called for placebo-controlled randomized clinical trials, which most in the field hold as unethical. His views on the efficacy of flu vaccines are rejected by medical institutions including the CDC and the National Institutes of Health, and by key figures in the field like Anthony Fauci.[284]
Michael Osterholm, who led the Center for Infectious Disease Research and Policy 2012 review on flu vaccines, recommended getting the vaccine but criticized its promotion, saying, "We have overpromoted and overhyped this vaccine ... it does not protect as promoted. It's all a sales job: it's all public relations."[285]
Veterinary use
[edit]Veterinary influenza vaccination aims to achieve the following four objectives:[286]
- Protection from clinical disease
- Protection from infection with virulent virus
- Protection from virus excretion
- Serological differentiation of infected from vaccinated animals (so-called DIVA principle).
Horses
[edit]Horses with horse flu can run a fever, have a dry hacking cough, have a runny nose, and become depressed and reluctant to eat or drink for several days but usually recover in two to three weeks. "Vaccination schedules generally require a primary course of two doses, 3–6 weeks apart, followed by boosters at 6–12 month intervals. It is generally recognized that in many cases such schedules may not maintain protective levels of antibody and more frequent administration is advised in high-risk situations."[287]
It is a common requirement at shows in the United Kingdom that horses be vaccinated against equine flu and a vaccination card must be produced; the International Federation for Equestrian Sports (FEI) requires vaccination every six months.[288][289]
Poultry
[edit]It is possible to vaccinate poultry against specific strains of highly pathogenic avian influenza. Vaccination should be combined with other control measures such as infection monitoring, early detection, and biosecurity.[290][291]
Pigs
[edit]Swine influenza vaccines are extensively used in pig farming in Europe and North America. Most swine flu vaccines include an H1N1 and an H3N2 strain.
Swine influenza has been recognized as a major problem since the outbreak in 1976. Evolution of the virus has resulted in inconsistent responses to traditional vaccines. Standard commercial swine flu vaccines are effective in controlling the problem when the virus strains match enough to have significant cross-protection. Customised (autogenous) vaccines made from the specific viruses isolated are made and used in the more difficult cases.[292] The vaccine manufacturer Novartis claims that the H3N2 strain (first identified in 1998) has brought major losses to pig farmers. Abortion storms are a common sign and sows stop eating for a few days and run a high fever. The mortality rate can be as high as fifteen percent.[293]
Dogs
[edit]In 2004, influenza A virus subtype H3N8 was discovered to cause canine influenza. Because of the lack of previous exposure to this virus, dogs have no natural immunity to this virus. However, a vaccine was found in 2004.[294]
Notes
[edit]- ^ (H1N1)pdm09 is newer nomenclature for the 2009 pandemic H1N1 virus, not a different strain.
References
[edit]- ^ a b "AusPAR: Influenza Haemagglutinin Recombinant". Therapeutic Goods Administration (TGA). August 23, 2021. Archived from the original on September 11, 2021. Retrieved September 10, 2021.
- ^ a b "AusPAR: Inactivated quadrivalent influenza vaccine (split virion) influenza virus haemagglutinin". Therapeutic Goods Administration (TGA). December 2, 2020. Archived from the original on September 11, 2021. Retrieved September 10, 2021.
- ^ "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). December 21, 2022. Archived from the original on April 3, 2022. Retrieved January 2, 2023.
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2017". Therapeutic Goods Administration (TGA). June 21, 2022. Archived from the original on April 10, 2023. Retrieved April 9, 2023.
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2016". Therapeutic Goods Administration (TGA). June 21, 2022. Archived from the original on April 10, 2023. Retrieved April 10, 2023.
- ^ https://www.tga.gov.au/resources/auspar/auspar-flucelvax-quad-0 [bare URL]
- ^ "Summary Basis of Decision (SBD) for Supemtek". Health Canada. October 23, 2014. Archived from the original on May 30, 2022. Retrieved May 29, 2022.
- ^ "Regulatory Decision Summary - Flucelvax Quad". Health Canada. October 23, 2014. Archived from the original on June 7, 2022. Retrieved June 7, 2022.
- ^ "Regulatory Decision Summary - Flucelvax Quad". Health Canada. October 23, 2014. Archived from the original on June 7, 2022. Retrieved June 7, 2022.
- ^ "Regulatory Decision Summary - Influvac Tetra". Health Canada. October 23, 2014. Archived from the original on June 7, 2022. Retrieved June 7, 2022.
- ^ "Regulatory Decision Summary for Panenza (Haemagglutinin-Strain A (H1N1))". Drug and Health Products Portal. October 27, 2023. Retrieved April 2, 2024.
- ^ "Afluria (influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (propiolactone inactivated), influenza a virus a/thailand/8/2022 ivr-237 (h3n2) antigen (propiolactone inactivated), influenza b virus b/austria/1359417/2021 bvr-26 antigen- propiolactone inactivated injection, suspension". DailyMed. July 1, 2024. Retrieved December 17, 2024.
- ^ "Audenz- influenza a virus h5n1 whole injection". DailyMed. August 6, 2024. Retrieved December 17, 2024.
- ^ "Fluad (influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/thailand/8/2022 ivr-237 (h3n2) antigen (formaldehyde inactivated), influenza b virus b/austria/1359417/2021 bvr-26 antigen- formaldehyde inactivated injection, suspension". DailyMed. July 1, 2024. Retrieved December 17, 2024.
- ^ "Fluarix 2024/2025- influenza virus vaccine suspension". DailyMed. July 1, 2024. Retrieved December 17, 2024.
- ^ "Flublok Trivalent Northern Hemisphere (influenza a virus a/west virginia/30/2022 (h1n1) recombinant hemagglutinin antigen, influenza a virus a/massachusetts/18/2022- h3n2 recombinant hemagglutinin antigen, and influenza b virus b/austria/1359417/2021 recombinant hemagglutinin antigen injection". DailyMed. October 18, 2024. Retrieved December 17, 2024.
- ^ "Flucelvax (influenza a virus a/georgia/12/2022 cvr-167 (h1n1) antigen (mdck cell derived, propiolactone inactivated), influenza a virus a/sydney/1304/2022 (h3n2) antigen (mdck cell derived, propiolactone inactivated), influenza b virus b/singapore/wuh4618/2021 antigen- mdck cell derived, propiolactone inactivated injection, suspension; Flucelvax (influenza a virus a/georgia/12/2022 crv-167 (h1n1) antigen (mdck cell derived, propiolactone inactivated), influenza a virus a/sydney/1304/2022 (h3n2) antigen (mdck cell derived, propiolactone inactivated), influenza b virus b/singapore/wuh4618/2021 antigen- mdck cell derived, propiolactone inactivated injection, suspension". DailyMed. July 1, 2024. Retrieved December 17, 2024.
- ^ "Flulaval 2024/2025- influenza virus vaccine suspension". DailyMed. July 1, 2024. Retrieved December 17, 2024.
- ^ "Flumist- influenza vaccine live intranasal spray". DailyMed. August 6, 2024. Retrieved December 17, 2024.
- ^ "Fluzone High-Dose Quadrivalent Northern Hemisphere (influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/california/122/2022 san-022 (h3n2) antigen (formaldehyde inactivated), influenza b virus b/phuket/3073/2013 antigen (formaldehyde inactivated), and influenza b virus b/michigan/01/2021 antigen- formaldehyde inactivated injection, suspension". DailyMed. July 30, 2024. Retrieved December 17, 2024.
- ^ "Fluzone Quadrivalent Northern Hemisphere (influenza a virus a/victoria/2570/2019 ivr-215 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/darwin/9/2021 san-010 (h3n2) antigen (formaldehyde inactivated), influenza b virus b/phuket/3073/2013 antigen (formaldehyde inactivated), and influenza b virus b/michigan/01/2021 antigen- formaldehyde inactivated injection, suspension". DailyMed. June 14, 2024. Retrieved December 17, 2024.
- ^ "Fluzone Quadrivalent Northern Hemisphere (influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/darwin/9/2021 san-010 (h3n2) antigen (formaldehyde inactivated), influenza b virus b/phuket/3073/2013 antigen (formaldehyde inactivated), and influenza b virus b/michigan/01/2021 antigen- formaldehyde inactivated injection, suspension; Flucelvax (influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/california/122/2022 san-022 (h3n2) antigen (formaldehyde inactivated), influenza b virus b/phuket/3073/2013 antigen (formaldehyde inactivated), and influenza b virus b/michigan/01/2021 antigen- formaldehyde inactivated injection, suspension". DailyMed. July 24, 2024. Retrieved December 17, 2024.
- ^ "Fluzone Quadrivalent Southern Hemisphere (influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/california/122/2022 (a/thailand/8/2022-like virus (h3n2) antigen (formaldehyde inactivated), influenza b virus b/michigan/01/2021 antigen (formaldehyde inactivated), and influenza b virus b/phuket/3073/2013 antigen- formaldehyde inactivated injection, suspension". DailyMed. February 22, 2024. Retrieved December 17, 2024.
- ^ "Supemtek EPAR". European Medicines Agency. November 25, 2020. Retrieved June 27, 2024.
- ^ "Fluad Tetra". European Medicines Agency (EMA). May 20, 2020. Retrieved August 10, 2024.
- ^ "Fluad Product information". Union Register of medicinal products. November 19, 2024. Retrieved December 17, 2024.
- ^ "Flucelvax Product information". Union Register of medicinal products. November 26, 2024. Retrieved December 17, 2024.
- ^ "Key Facts About Seasonal Flu Vaccine". Influenza (Flu). September 30, 2024.
- ^ "The flu jab in pregnancy". nhs.uk. December 3, 2020.
- ^ a b c d e f g h i World Health Organization (November 2012). "Vaccines against influenza WHO position paper". Weekly Epidemiological Record. 87 (47): 461–76. hdl:10665/241993. PMID 23210147.
- ^ World Health Organization (May 2022). "Vaccines against influenza: WHO position paper – May 2022". Weekly Epidemiological Record. 97 (19): 185–208. hdl:10665/354265.
- ^ Manzoli L, Ioannidis JP, Flacco ME, De Vito C, Villari P (July 2012). "Effectiveness and harms of seasonal and pandemic influenza vaccines in children, adults and elderly: a critical review and re-analysis of 15 meta-analyses". Human Vaccines & Immunotherapeutics. 8 (7): 851–62. doi:10.4161/hv.19917. PMC 3495721. PMID 22777099.
- ^ Compans RW (2009). Vaccines for pandemic influenza. Dordrecht: Springer. p. 49. ISBN 978-3-540-92165-3. Archived from the original on August 3, 2020. Retrieved September 9, 2017.
- ^ Vaccine Analysis: Strategies, Principles, and Control. Springer. 2014. p. 61. ISBN 978-3-662-45024-6. Archived from the original on August 3, 2020. Retrieved September 9, 2017.
- ^ a b c d "Who Should and Who Should NOT get a Flu Vaccine". U.S. Centers for Disease Control and Prevention (CDC). October 11, 2019. Archived from the original on December 2, 2019. Retrieved December 2, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ The immunological basis for immunization series: module 23: influenza vaccines. World Health Organization (WHO). October 2017. hdl:10665/259211. ISBN 978-92-4-151305-0.
- ^ a b c d e f g h i j k l m n o p q r s Grohskopf LA, Alyanak E, Ferdinands JM, Broder KR, Blanton LH, Talbot HK, et al. (August 2021). "Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021-22 Influenza Season" (PDF). MMWR Recomm Rep. 70 (5): 1–28. doi:10.15585/mmwr.rr7005a1. PMC 8407757. PMID 34448800. Archived (PDF) from the original on August 27, 2021. Retrieved August 29, 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "Implementation of the Council Recommendation on seasonal influenza vaccination (2009/1019/EU)" (PDF). European Centre for Disease Prevention and Control. January 2014. Archived (PDF) from the original on April 10, 2020. Retrieved April 10, 2020.
- Lay summary in: "Implementation of the Council Recommendation on seasonal influenza vaccination". European Centre for Disease Prevention and Control. January 9, 2014.
- ^ a b c d e "Flu Vaccine and People with Egg Allergies". U.S. Centers for Disease Control and Prevention (CDC). November 25, 2019. Archived from the original on December 2, 2019. Retrieved December 2, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Intradermal Influenza (Flu) Vaccination". U.S. Centers for Disease Control and Prevention (CDC). October 31, 2018. Archived from the original on October 14, 2019. Retrieved October 14, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Influenza vaccines – United States, 2019–20 influenza season". U.S. Centers for Disease Control and Prevention (CDC). August 22, 2019. Archived from the original on October 14, 2019. Retrieved October 14, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Influenza Virus Vaccine Inactivated". The American Society of Health-System Pharmacists. November 19, 2018. Archived from the original on October 14, 2019. Retrieved October 13, 2019.
- ^ Institute of Medicine (2005). Knobler SL, Mack A, Mahmoud A, Lemon SM (eds.). The Threat of Pandemic Influenza: Are We Ready? Workshop Summary. The National Academies Press. p. 62. doi:10.17226/11150. ISBN 978-0-309-09504-4. PMID 20669448.
- ^ Plotkin, S.L. and Plotkin, S.A. "A short history of vaccination". In: Vaccines, Stanley A. Plotkin, Walter A. Orenstein, Paul A. Offit, eds. Elsevier Health Sciences, 2008, pp. 6–7.
- ^ Artenstein, A. W. "Influenza" In: Vaccines: A Biography, Andrew W. Artenstein, ed. pp. 191–205.
- ^ Hampson AW (June 2008). "Vaccines for pandemic influenza. The history of our current vaccines, their limitations and the requirements to deal with a pandemic threat". Annals of the Academy of Medicine, Singapore. 37 (6): 510–17. doi:10.47102/annals-acadmedsg.V37N6p510. PMID 18618064. S2CID 17102174.
- ^ Milián E, Kamen AA (2015). "Current and emerging cell culture manufacturing technologies for influenza vaccines". Biomed Res Int. 2015: 504831. doi:10.1155/2015/504831. PMC 4359798. PMID 25815321.
- ^ a b "FDA approves first seasonal influenza vaccine manufactured using cell culture technology" (Press release). U.S. Food and Drug Administration (FDA). Archived from the original on January 2, 2013.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Cell-Based Flu Vaccines". U.S. Centers for Disease Control and Prevention (CDC). October 11, 2019. Archived from the original on December 2, 2019. Retrieved December 2, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "FDA approves new seasonal influenza vaccine made using novel technology" (Press release). U.S. Food and Drug Administration (FDA). January 16, 2013. Archived from the original on May 18, 2013.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Landry N, Ward BJ, Trépanier S, Montomoli E, Dargis M, Lapini G, et al. (December 2010). "Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza". PLOS ONE. 5 (12): e15559. Bibcode:2010PLoSO...515559L. doi:10.1371/journal.pone.0015559. PMC 3008737. PMID 21203523.
- ^ Osterholm MT (May 2005). "Preparing for the next pandemic". The New England Journal of Medicine. 352 (18): 1839–42. CiteSeerX 10.1.1.608.6200. doi:10.1056/NEJMp058068. PMID 15872196. S2CID 45893174.
- ^ "Swine Flu Epidemics". October 9, 1999. Archived from the original on October 9, 1999.
- ^ McCullers JA, Van De Velde LA, Allison KJ, Branum KC, Webby RJ, Flynn PM (June 2010). "Recipients of vaccine against the 1976 "swine flu" have enhanced neutralization responses to the 2009 novel H1N1 influenza virus". Clinical Infectious Diseases. 50 (11): 1487–92. doi:10.1086/652441. PMC 2946351. PMID 20415539.
- ^ a b Doshi P (September 20, 2018). "Pandemrix vaccine: why was the public not told of early warning signs?". BMJ. 362: k3948. doi:10.1136/bmj.k3948. ISSN 0959-8138. S2CID 52308748.
- ^ a b Doshi P (September 20, 2018). "Pandemrix vaccine: why was the public not told of early warning signs?". BMJ. 362: Infographic. doi:10.1136/bmj.k3948. S2CID 52308748.
- ^ Cohet C, van der Most R, Bauchau V, Bekkat-Berkani R, Doherty TM, Schuind A, et al. (May 2019). "Safety of AS03-adjuvanted influenza vaccines: A review of the evidence". Vaccine. 37 (23): 3006–3021. doi:10.1016/j.vaccine.2019.04.048. PMID 31031030.
- ^ "First Quadrivalent Vaccine Against Seasonal Flu Wins FDA Approval". March 2, 2012. Archived from the original on March 4, 2012.
- ^ "FDA approves first quadrivalent vaccine to prevent seasonal influenza" (Press release). U.S. Food and Drug Administration (FDA). Archived from the original on December 21, 2012.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "December 14, 2012 Approval Letter – Fluarix Quadrivalent". U.S. Food and Drug Administration (FDA). Archived from the original on January 2, 2013.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ National Advisory Committee on Immunization (NACI) (July 2014). Literature review on quadrivalent influenza vaccines (PDF). Ottawa: Public Health Agency of Canada. ISBN 978-1-100-24682-6. Cat.: HP40-117/2014E-PDF Pub.: 140118. Archived (PDF) from the original on August 1, 2020. Retrieved January 11, 2020.
- ^ "What You Should Know for the 2018-2019 Influenza Season". Centers for Disease Control and Prevention (CDC). January 10, 2019. Archived from the original on August 6, 2020. Retrieved February 5, 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c d e f g "Frequently Asked Influenza (Flu) Questions: 2019–2020 Season". U.S. Centers for Disease Control and Prevention (CDC). November 5, 2019. Archived from the original on December 1, 2019. Retrieved November 30, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Fluzone High-Dose Quadrivalent". U.S. Food and Drug Administration (FDA). November 4, 2019. STN: BL 103914. Archived from the original on January 12, 2020. Retrieved February 5, 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "FDA approves Fluzone High-Dose Quadrivalent (Influenza Vaccine) for adults 65 years of age and older". Sanofi (Press release). November 4, 2019. Archived from the original on August 1, 2020. Retrieved February 5, 2020.
- ^ a b c "Fluad Quadrivalent". U.S. Food and Drug Administration (FDA). July 2, 2020. STN: 125510. Archived from the original on August 11, 2020. Retrieved August 25, 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Seqirus Receives FDA Approval for Fluad Quadrivalent for Adults 65 Years and Older". Seqirus (Press release). February 24, 2020. Archived from the original on August 26, 2020. Retrieved August 25, 2020.
- ^ "Seqirus Begins Shipping 2020/21 Influenza Vaccines to U.S. Market". Seqirus (Press release). July 30, 2020. Archived from the original on August 26, 2020. Retrieved August 25, 2020.
- ^ Koutsakos M, Wheatley AK, Laurie K, Kent SJ, Rockman S (December 2021). "Influenza lineage extinction during the COVID-19 pandemic?". Nature Reviews. Microbiology. 19 (12): 741–742. doi:10.1038/s41579-021-00642-4. PMC 8477979. PMID 34584246.
- ^ a b World Health Organization (September 29, 2023). "Questions and Answers: Recommended composition of influenza virus vaccines for use in the southern hemisphere 2024 influenza season and development of candidate vaccine viruses for pandemic preparedness" (PDF). Archived (PDF) from the original on October 10, 2023. Retrieved October 26, 2023.
- ^ a b Schnirring L (September 29, 2023). "WHO advisers recommend switch back to trivalent flu vaccines". CIDRAP. Archived from the original on December 18, 2023. Retrieved October 26, 2023.
- ^ a b "Use of Trivalent Influenza Vaccines for the 2024-2025 U.S. flu season". U.S. Food and Drug Administration (FDA). March 5, 2024. Archived from the original on March 7, 2024. Retrieved March 7, 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Flucelvax (influenza a virus a/georgia/12/2022 cvr-167 (h1n1) antigen (mdck cell derived, propiolactone inactivated), influenza a virus a/sydney/1304/2022 (h3n2) antigen (mdck cell derived, propiolactone inactivated), influenza b virus b/singapore/wuh4618/2021 antigen- mdck cell derived, propiolactone inactivated injection, suspension; Flucelvax (influenza a virus a/georgia/12/2022 crv-167 (h1n1) antigen (mdck cell derived, propiolactone inactivated), influenza a virus a/sydney/1304/2022 (h3n2) antigen (mdck cell derived, propiolactone inactivated), influenza b virus b/singapore/wuh4618/2021 antigen- mdck cell derived, propiolactone inactivated injection, suspension". DailyMed. July 1, 2024. Retrieved August 31, 2024.
- ^ "Fluad (influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/thailand/8/2022 ivr-237 (h3n2) antigen (formaldehyde inactivated), influenza b virus b/austria/1359417/2021 bvr-26 antigen- formaldehyde inactivated injection, suspension". DailyMed. July 1, 2024. Retrieved August 31, 2024.
- ^ "Fluzone High-Dose Quadrivalent Northern Hemisphere (influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/california/122/2022 san-022 (h3n2) antigen (formaldehyde inactivated), influenza b virus b/phuket/3073/2013 antigen (formaldehyde inactivated), and influenza b virus b/michigan/01/2021 antigen- formaldehyde inactivated injection, suspension". DailyMed. July 30, 2024. Retrieved August 31, 2024.
- ^ a b c d e f "Key Facts About Seasonal Flu Vaccine". U.S. Centers for Disease Control and Prevention (CDC). December 2, 2019. Archived from the original on December 2, 2019. Retrieved December 2, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "Flu vaccine". UK National Health Service. May 13, 2022. Archived from the original on October 26, 2021. Retrieved August 13, 2022.
- ^ Osterholm MT, Kelley NS, Sommer A, Belongia EA (January 2012). "Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis". The Lancet. Infectious Diseases. 12 (1): 36–44. doi:10.1016/s1473-3099(11)70295-x. PMID 22032844.
- ^ MacIntyre CR, Mahimbo A, Moa AM, Barnes M (December 2016). "Influenza vaccine as a coronary intervention for prevention of myocardial infarction". Heart. 102 (24): 1953–1956. doi:10.1136/heartjnl-2016-309983. PMC 5256393. PMID 27686519.
- ^ "Past Seasons Vaccine Effectiveness Estimates". U.S. Centers for Disease Control and Prevention (CDC). January 29, 2020. Archived from the original on February 12, 2020. Retrieved March 4, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Centers for Disease Control Prevention (CDC) (August 13, 2004). "Assessment of the effectiveness of the 2003-04 influenza vaccine among children and adults--Colorado, 2003". MMWR. Morbidity and Mortality Weekly Report. 53 (31). Centers for Disease Control and Prevention: 707–710. ISSN 1545-861X. PMID 15306754. Archived from the original on April 27, 2022. Retrieved April 27, 2022. At table.
- ^ Centers for Disease Control Prevention (CDC) (January 16, 2004). "Preliminary assessment of the effectiveness of the 2003-04 inactivated influenza vaccine--Colorado, December 2003". MMWR. Morbidity and Mortality Weekly Report. 53 (1). Centers for Disease Control and Prevention: 8–11. ISSN 1545-861X. PMID 14724559. Archived from the original on May 29, 2022. Retrieved April 25, 2022.
- ^ "Past Weekly Surveillance Reports". Centers for Disease Control and Prevention. April 29, 2022. Archived from the original on April 22, 2022. Retrieved April 25, 2022.
- ^ Chung JR (March 11, 2022). "Interim Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness — United States, February 2022". MMWR. Morbidity and Mortality Weekly Report. 71 (10): 365–370. doi:10.15585/mmwr.mm7110a1. ISSN 0149-2195. PMC 8911998. PMID 35271561. Archived from the original on May 16, 2022. Retrieved May 25, 2022.
- ^ Dreisbach EN (June 3, 2021). "CDC unable to estimate flu vaccine effectiveness after historically mild season". Healio. Archived from the original on October 8, 2021. Retrieved April 18, 2022.
- ^ Fedson DS (1998). "Measuring protection: efficacy versus effectiveness". Developments in Biological Standardization. 95: 195–201. PMID 9855432.
- ^ a b c d e Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C (February 2018). "Vaccines for preventing influenza in healthy adults". Cochrane Database of Systematic Reviews. 2020 (2): CD001269. doi:10.1002/14651858.CD001269.pub6. PMC 6491184. PMID 29388196.
- ^ Jefferson T (October 2006). "Influenza vaccination: policy versus evidence". BMJ. 333 (7574): 912–15. doi:10.1136/bmj.38995.531701.80. PMC 1626345. PMID 17068038.
- ^ "2007–2008 Influenza (Flu) Season". U.S. Centers for Disease Control and Prevention (CDC). June 26, 2008. Archived from the original on March 6, 2008.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Carrat F, Flahault A (September 2007). "Influenza vaccine: the challenge of antigenic drift". Vaccine. 25 (39–40): 6852–6862. doi:10.1016/j.vaccine.2007.07.027. PMID 17719149.
- ^ Sautto GA, Kirchenbaum GA, Ross TM (January 19, 2018). "Towards a universal influenza vaccine: different approaches for one goal". Virology Journal. 15 (1): 17. doi:10.1186/s12985-017-0918-y. PMC 5785881. PMID 29370862.
- ^ a b Chow EJ, Doyle JD, Uyeki TM (June 12, 2019). "Influenza virus-related critical illness: prevention, diagnosis, treatment". Critical Care. 23 (1): 214. doi:10.1186/s13054-019-2491-9. PMC 6563376. PMID 31189475.
- ^ Krammer F, Smith GJ, Fouchier RA, Peiris M, Kedzierska K, Doherty PC, et al. (June 28, 2018). "Influenza". Nature Reviews Disease Primers. 4 (1): 3. doi:10.1038/s41572-018-0002-y. PMC 7097467. PMID 29955068.
- ^ Dabestani NM, Leidner AJ, Seiber EE, Kim H, Graitcer SB, Foppa IM, et al. (September 2019). "A review of the cost-effectiveness of adult influenza vaccination and other preventive services". Preventive Medicine. 126: 105734. doi:10.1016/j.ypmed.2019.05.022. PMC 6778688. PMID 31152830.
- ^ a b c Ghebrehewet S, MacPherson P, Ho A (December 7, 2016). "Influenza". The BMJ. 355: i6258. doi:10.1136/bmj.i6258. PMC 5141587. PMID 27927672.
- ^ a b Principi N, Esposito S (March 4, 2018). "Protection of children against influenza: Emerging problems". Human Vaccines & Immunotherapeutics. 14 (3): 750–757. doi:10.1080/21645515.2017.1279772. PMC 5861800. PMID 28129049.
- ^ Thompson MG, Pierse N, Huang QS, Prasad N, Duque J, Newbern EC, et al. (September 18, 2018). "Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012–2015". Vaccine. 36 (39): 5916–5925. doi:10.1016/j.vaccine.2018.07.028. ISSN 0264-410X. PMID 30077480. S2CID 51922011. Archived from the original on May 27, 2022. Retrieved April 23, 2022.
- ^ Tenforde MW, Talbot HK, Trabue CH, Gaglani M, McNeal TS, Monto AS, et al. (December 30, 2020). "Influenza Vaccine Effectiveness Against Hospitalization in the United States, 2019–2020". The Journal of Infectious Diseases. 224 (5): 813–820. doi:10.1093/infdis/jiaa800. ISSN 0022-1899. PMC 8408767. PMID 33378531. Archived from the original on April 23, 2022. Retrieved April 23, 2022.
- ^ Ferdinands JM, Thompson MG, Blanton L, Spencer S, Grant L, Fry AM (June 23, 2021). "Does influenza vaccination attenuate the severity of breakthrough infections? A narrative review and recommendations for further research". Vaccine. 39 (28): 3678–3695. doi:10.1016/j.vaccine.2021.05.011. ISSN 0264-410X. PMID 34090700. S2CID 235361401. Archived from the original on April 23, 2022. Retrieved April 23, 2022.
- ^ a b c "Vaccine Effectiveness: How Well Do the Flu Vaccines Work?". U.S. Centers for Disease Control and Prevention (CDC). October 12, 2018. Archived from the original on October 25, 2019. Retrieved October 24, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Ramsay LC, Buchan SA, Stirling RG, Cowling BJ, Feng S, Kwong JC, et al. (January 2019). "The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis". BMC Med. 17 (1): 9. doi:10.1186/s12916-018-1239-8. PMC 6327561. PMID 30626399.
- ^ Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G (July 2017). "Repeated annual influenza vaccination and vaccine effectiveness: review of evidence". Expert Rev Vaccines. 16 (7): 723–36. doi:10.1080/14760584.2017.1334554. PMID 28562111.
- ^ Gemmill I, Young K (June 7, 2018). "Summary of the NACI literature review on the comparative effectiveness of subunit and split virus inactivated influenza vaccines in older adults". Canada Communicable Disease Report. 44 (6): 129–133. doi:10.14745/ccdr.v44i06a02. ISSN 1481-8531. PMC 6449119. PMID 31015805. Archived from the original on May 17, 2020. Retrieved June 2, 2020.
- ^ "Flu & People 65 Years and Older". Centers for Disease Control and Prevention. August 26, 2021. Archived from the original on April 22, 2022. Retrieved April 21, 2022.
- ^ MacIntyre CR, Mahimbo A, Moa AM, Barnes M (December 2016). "Influenza vaccine as a coronary intervention for prevention of myocardial infarction". Heart. 102 (24): 1953–1956. doi:10.1136/heartjnl-2016-309983. PMC 5256393. PMID 27686519.
- ^ Zeno EE (2024). "Interim Effectiveness Estimates of 2024 Southern Hemisphere Influenza Vaccines in Preventing Influenza-Associated Hospitalization — REVELAC-i Network, Five South American Countries, March–July 2024". MMWR. Morbidity and Mortality Weekly Report. 73 (39): 861–868. doi:10.15585/mmwr.mm7339a1. ISSN 0149-2195. PMC 11449270. PMID 39361525.
- ^ Benadjaoud Y. "Flu vaccine lowered risk of hospitalization in Southern Hemisphere by 35%: CDC". ABC News. Retrieved October 4, 2024.
- ^ a b "Influenza Historic Timeline | Pandemic Influenza (Flu) | CDC". March 11, 2020. Archived from the original on January 30, 2022. Retrieved January 27, 2022.
- ^ a b "Influenza (Seasonal)". World Health Organization (WHO). November 6, 2018. Archived from the original on October 25, 2019. Retrieved October 24, 2019.
- ^ "Study of Flu-Related Deaths in Children Shows Healthy Children at Risk". U.S. Centers for Disease Control and Prevention (CDC). February 12, 2018. Archived from the original on December 2, 2019. Retrieved December 2, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V (February 2018). "Vaccines for preventing influenza in healthy children". Cochrane Database of Systematic Reviews. 2018 (2): CD004879. doi:10.1002/14651858.CD004879.pub5. PMC 6491174. PMID 29388195.
- ^ Steenhuysen J (January 22, 2018). "U.S. CDC director urges flu vaccinations as pediatric deaths mount". Reuters. Archived from the original on May 6, 2021. Retrieved January 26, 2018.
- ^ "Fluzone High-Dose Quadrivalent". U.S. Food and Drug Administration (FDA). November 14, 2019. Archived from the original on December 1, 2019. Retrieved November 30, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Afluria Quadrivalent". U.S. Food and Drug Administration (FDA). November 8, 2019. Archived from the original on December 1, 2019. Retrieved November 30, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ National Advisory Committee on Immunization (NACI) (November 2018). "Supplemental Statement – Afluria Tetra – An Advisory Committee Statement (ACS)". Public Health Agency of Canada. Ottawa. Cat.: HP37-25E-PDF Pub.: 180566. Archived from the original on January 12, 2020. Retrieved January 11, 2020.
- ^ Literature review on influenza vaccination in healthy 5–18-year-olds (PDF) (Report). Ottawa: Public Health Agency of Canada. July 2014. ISBN 978-1-100-24681-9. Cat.: HP40-116/2014E-PDF Pub.: 140116. Archived (PDF) from the original on August 1, 2020. Retrieved January 12, 2020.
- ^ Literature Review on Pediatric Fluad Influenza Vaccine Use in Children 6–72 Months of Age (PDF) (Report). Ottawa: Public Health Agency of Canada. 2015. Archived (PDF) from the original on May 17, 2020. Retrieved January 11, 2020.
- ^ Peleg N, Zevit N, Shamir R, Chodick G, Levy I (January 2015). "Seasonal influenza vaccination rates and reasons for non-vaccination in children with gastrointestinal disorders". Vaccine. 33 (1): 182–186. doi:10.1016/j.vaccine.2014.10.086. PMID 25444802.
- ^ a b Osterholm MT, Kelley NS, Sommer A, Belongia EA (January 2012). "Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis". The Lancet. Infectious Diseases. 12 (1): 36–44. doi:10.1016/S1473-3099(11)70295-X. PMID 22032844.
- ^ a b Burls A, Jordan R, Barton P, Olowokure B, Wake B, Albon E, et al. (May 2006). "Vaccinating healthcare workers against influenza to protect the vulnerable – is it a good use of healthcare resources? A systematic review of the evidence and an economic evaluation". Vaccine. 24 (19): 4212–21. doi:10.1016/j.vaccine.2005.12.043. PMID 16546308.
- ^ Ahmed F, Lindley MC, Allred N, Weinbaum CM, Grohskopf L (January 2014). "Effect of influenza vaccination of healthcare personnel on morbidity and mortality among patients: systematic review and grading of evidence". Clinical Infectious Diseases. 58 (1): 50–57. doi:10.1093/cid/cit580. PMID 24046301.
- ^ Griffin MR (January 2014). "Influenza vaccination of healthcare workers: making the grade for action". Clinical Infectious Diseases. 58 (1): 58–60. doi:10.1093/cid/cit590. PMID 24046312.
- ^ Simonsen L, Viboud C, Taylor RJ, Miller MA, Jackson L (October 2009). "Influenza vaccination and mortality benefits: new insights, new opportunities". Vaccine. 27 (45): 6300–04. doi:10.1016/j.vaccine.2009.07.008. PMID 19840664.
- ^ Demicheli V, Jefferson T, Di Pietrantonj C, Ferroni E, Thorning S, Thomas RE, et al. (February 2018). "Vaccines for preventing influenza in the elderly". Cochrane Database of Systematic Reviews. 2 (11): CD004876. doi:10.1002/14651858.CD004876.pub4. PMC 6491101. PMID 29388197.
- ^ Darvishian M, Bijlsma MJ, Hak E, van den Heuvel ER (December 2014). "Effectiveness of seasonal influenza vaccine in community-dwelling elderly people: a meta-analysis of test-negative design case-control studies". The Lancet. Infectious Diseases. 14 (12): 1228–39. doi:10.1016/S1473-3099(14)70960-0. PMID 25455990.
- ^ Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E (October 2007). "Effectiveness of influenza vaccine in the community-dwelling elderly". The New England Journal of Medicine. 357 (14): 1373–81. doi:10.1056/NEJMoa070844. PMID 17914038. S2CID 14850833.
- ^ Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA (October 2007). "Mortality benefits of influenza vaccination in elderly people: an ongoing controversy". The Lancet. Infectious Diseases. 7 (10): 658–66. doi:10.1016/S1473-3099(07)70236-0. PMID 17897608.
- ^ Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. (January 2003). "Mortality associated with influenza and respiratory syncytial virus in the United States". JAMA. 289 (2): 179–86. doi:10.1001/jama.289.2.179. PMID 12517228. S2CID 5018362.
- ^ "High Dose Flu Vaccine for the Elderly « Science-Based Medicine". Sciencebasedmedicine.org. October 19, 2010. Archived from the original on May 8, 2013. Retrieved October 17, 2013.
- ^ "Fluzone High-Dose Seasonal Influenza Vaccine". U.S. Centers for Disease Control and Prevention (CDC). September 6, 2019. Archived from the original on December 2, 2019. Retrieved December 2, 2019.
- ^ DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, et al. (August 2014). "Efficacy of high-dose versus standard-dose influenza vaccine in older adults". The New England Journal of Medicine. 371 (7): 635–45. doi:10.1056/NEJMoa1315727. PMID 25119609. S2CID 205096393.
- ^ Wells C, Grobelna A (January 8, 2019). "High Dose Influenza Vaccine for Adults: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines" (PDF). Rapid Response Report. Ottawa: Canadian Agency for Drugs and Technologies in Health (CADTH). ISSN 1922-8147. PMID 31141324. Archived (PDF) from the original on August 1, 2020. Retrieved August 15, 2022.
- ^ Mascagni P, Vicenzi E, Kajaste-Rudnitski A, Pellicciotta G, Monti A, Cervi C, et al. (2012). "Assessment of efficacy and safety of pandemic A/H1N1/2009 influenza vaccine in a group of health care workers". La Medicina del Lavoro. 103 (3): 220–29. PMID 22838300.
- ^ "FDA approves first seasonal influenza vaccine containing an adjuvant" (Press release). U.S. Food and Drug Administration (FDA). November 24, 2015. Archived from the original on July 22, 2017. Retrieved August 20, 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "Flu Vaccine With Adjuvant". U.S. Centers for Disease Control and Prevention (CDC). September 4, 2019. Archived from the original on December 2, 2019. Retrieved December 2, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Fluad". U.S. Food and Drug Administration (FDA). November 8, 2019. STN 125510. Archived from the original on December 2, 2019. Retrieved December 2, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Influenza vaccine with squalene adjuvant: new preparation. No better than available products". Prescrire International. 13 (74): 206–08. December 2004. PMID 15599987.
- ^ Camilloni B, Basileo M, Valente S, Nunzi E, Iorio AM (2015). "Immunogenicity of intramuscular MF59-adjuvanted and intradermal administered influenza enhanced vaccines in subjects aged over 60: A literature review". Human Vaccines & Immunotherapeutics. 11 (3): 553–63. doi:10.1080/21645515.2015.1011562. PMC 4514405. PMID 25714138.
- Lay summary in: "Literature Review Update on the Efficacy and Effectiveness of High-Dose (Fluzone High-Dose) and MF59-Adjuvanted (Fluad) Trivalent Inactivated Influenza Vaccines in Adults 65 Years of Age and Older". Public Health Agency of Canada. May 1, 2018.
- ^ Van Damme P, Arnou R, Kafeja F, Fiquet A, Richard P, Thomas S, et al. (May 2010). "Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomised comparative study". BMC Infectious Diseases. 10: 134. doi:10.1186/1471-2334-10-134. PMC 2895601. PMID 20504306.
- ^ Haverkate M, D'Ancona F, Giambi C, Johansen K, Lopalco PL, Cozza V, et al. (VENICE project gatekeepers contact points collective) (May 2012). "Mandatory and recommended vaccination in the EU, Iceland, and Norway: results of the VENICE 2010 survey on the ways of implementing national vaccination programmes". Euro Surveillance. 17 (22). doi:10.2807/ese.17.22.20183-en. PMID 22687916.
- ^ Field RI (November 2009). "Mandatory vaccination of health care workers: whose rights should come first?". P & T. 34 (11): 615–18. PMC 2810172. PMID 20140133.
- ^ Kassianos G (2015). "Willingness of European healthcare workers to undergo vaccination against seasonal influenza: current situation and suggestions for improvement". Drugs in Context. 4: 212268. doi:10.7573/dic.212268. PMC 4316812. PMID 25657810.
- ^ Thomas RE, Jefferson T, Lasserson TJ (June 2016). "Influenza vaccination for healthcare workers who care for people aged 60 or older living in long-term care institutions". The Cochrane Database of Systematic Reviews. 2016 (6): CD005187. doi:10.1002/14651858.CD005187.pub5. PMC 8504984. PMID 27251461.
- ^ "Fluad Quad Australian prescription medicine decision summary". Therapeutic Goods Administration (TGA). December 13, 2019. Archived from the original on March 8, 2020. Retrieved August 24, 2020.
- ^ "Fluad Tetra EPAR". European Medicines Agency (EMA). March 24, 2020. Archived from the original on August 1, 2020. Retrieved May 29, 2020.
- ^ "Fluad Tetra Product information". Union Register of medicinal products. Archived from the original on March 5, 2023. Retrieved March 3, 2023.
- ^ Fell DB, Sprague AE, Liu N, Yasseen AS, Wen SW, Smith G, et al. (June 2012). "H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes". American Journal of Public Health (Submitted manuscript). 102 (6): e33–40. doi:10.2105/AJPH.2011.300606. PMC 3483960. PMID 22515877.
- ^ Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, et al. (September 2014). "Influenza vaccination of pregnant women and protection of their infants". The New England Journal of Medicine. 371 (10): 918–31. doi:10.1056/NEJMoa1401480. hdl:2263/42412. PMID 25184864.
- ^ "Flu shot". CDC. 2020. Archived from the original on December 2, 2019. Retrieved September 24, 2020.
- ^ "Misconceptions about flu vaccines". CDC. 2020. Archived from the original on August 10, 2020. Retrieved September 24, 2020.
- ^ McNeil Jr DG (October 1, 2018). "Over 80,000 Americans Died of Flu Last Winter, Highest Toll in Years". The New York Times. Archived from the original on October 1, 2018. Retrieved June 24, 2024.
- ^ "5 myths about the flu vaccine". World Health Organization. Archived from the original on February 14, 2024. Retrieved June 24, 2024.
- ^ Haber P, Sejvar J, Mikaeloff Y, DeStefano F (2009). "Vaccines and Guillain-Barré syndrome". Drug Safety. 32 (4): 309–23. doi:10.2165/00002018-200932040-00005. PMID 19388722. S2CID 33670594.
- ^ "Reorganized text". JAMA Otolaryngology–Head & Neck Surgery. 141 (5): 428. May 2015. doi:10.1001/jamaoto.2015.0540. PMID 25996397. S2CID 26612829.
- ^ Stowe J, Andrews N, Wise L, Miller E (February 2009). "Investigation of the temporal association of Guillain-Barre syndrome with influenza vaccine and influenza-like illness using the United Kingdom General Practice Research Database". American Journal of Epidemiology. 169 (3): 382–88. doi:10.1093/aje/kwn310. PMID 19033158.
- ^ a b Sivadon-Tardy V, Orlikowski D, Porcher R, Sharshar T, Durand MC, Enouf V, et al. (January 2009). "Guillain-Barré syndrome and influenza virus infection". Clinical Infectious Diseases. 48 (1): 48–56. doi:10.1086/594124. PMID 19025491.
- ^ Vellozzi C, Burwen DR, Dobardzic A, Ball R, Walton K, Haber P (March 2009). "Safety of trivalent inactivated influenza vaccines in adults: background for pandemic influenza vaccine safety monitoring". Vaccine. 27 (15): 2114–20. doi:10.1016/j.vaccine.2009.01.125. PMID 19356614. Archived from the original on August 1, 2020. Retrieved May 21, 2020.
- ^ Reinberg S (February 2, 2011). "Last Year's H1N1 Flu Vaccine Was Safe, Study Finds". U.S. News & World Report. Archived from the original on April 25, 2013.
- ^ a b c d Roberts L (March 27, 2021). "Australia's first cell-based influenza vaccines to roll out this flu season". ABC News. Archived from the original on April 27, 2021. Retrieved April 28, 2021.
- ^ National Advisory Committee on Immunization (NACI) (August 2012). "Statement on Seasonal Influenza Vaccine for 2012–2013" (PDF). Canada Communicable Disease Report. 38. Ottawa. Archived from the original (PDF) on January 17, 2013. Retrieved July 18, 2013.
- ^ Turner PJ, Southern J, Andrews NJ, Miller E, Erlewyn-Lajeunesse M (December 2015). "Safety of live attenuated influenza vaccine in young people with egg allergy: multicentre prospective cohort study". BMJ. 351: h6291. doi:10.1136/bmj.h6291. PMC 4673102. PMID 26645895.
- ^ Greenhawt M (December 2015). "Live attenuated influenza vaccine for children with egg allergy". BMJ. 351: h6656. doi:10.1136/bmj.h6656. PMID 26657778. S2CID 37037904.
- ^ a b "Novartis receives FDA approval for Flucelvax, the first cell-culture vaccine in US to help protect against seasonal influenza" (Press release). Novartis. November 20, 2012. Archived from the original on November 28, 2012.
- ^ a b "Supemtek EPAR". European Medicines Agency (EMA). September 15, 2020. Archived from the original on January 10, 2021. Retrieved November 27, 2020.
- ^ Technical Report: Narcolepsy in association with pandemic influenza vaccination (PDF). Stockholm, Sweden: European Centre for Disease Prevention and Control (ECDC). 2012. ISBN 978-92-9193-388-4. Archived from the original (PDF) on December 31, 2013. Retrieved December 30, 2013.
- ^ Yong E (2013). "Narcolepsy confirmed as autoimmune disease". Nature. doi:10.1038/nature.2013.14413. S2CID 74850662.
- ^ "Thimerosal in Flu Vaccine". U.S. Centers for Disease Control and Prevention. October 16, 2015. Archived from the original on December 2, 2019. Retrieved December 2, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Thimerosal in Vaccines Thimerosal – Concerns – Vaccine Safety". U.S. Centers for Disease Control and Prevention. October 27, 2015. Archived from the original on November 2, 2019. Retrieved December 2, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Offit PA (September 2007). "Thimerosal and vaccines – a cautionary tale". The New England Journal of Medicine. 357 (13): 1278–79. doi:10.1056/NEJMp078187. PMID 17898096. S2CID 36318722.
- ^ Global Advisory Committee on Vaccine Safety (July 14, 2006). "Thiomersal and vaccines". World Health Organization (WHO). Archived from the original on November 6, 2009. Retrieved November 20, 2007.
- ^ Grande AJ, Reid H, Thomas EE, Nunan D, Foster C (August 2016). "Exercise prior to influenza vaccination for limiting influenza incidence and its related complications in adults". The Cochrane Database of Systematic Reviews. 2016 (8): CD011857. doi:10.1002/14651858.CD011857.pub2. PMC 8504432. PMID 27545762.
- ^ Plotkin SA, Orenstein WA (1988). Vaccines. Philadelphia: W.B. Saunders Company. p. 424. ISBN 978-0-7216-1946-0. Retrieved September 7, 2020.
- ^ Product Monograph: Flumist (PDF), Astrazeneca Canada Inc., June 20, 2014, archived (PDF) from the original on September 30, 2020, retrieved September 5, 2020
- ^ "Global Influenza Surveillance and Response System (GISRS)". World Health Organization. Archived from the original on October 3, 2011. Retrieved October 22, 2019.
- ^ a b Schnirring L (September 29, 2023). "WHO advisers recommend switch back to trivalent flu vaccines". Center for Infectious Disease Research and Policy. Archived from the original on December 18, 2023. Retrieved January 23, 2024.
- ^ "Spotlight: Influenza". World Health Organization. Archived from the original on October 18, 2019. Retrieved October 22, 2019.
- ^ "Global influenza surveillance". WHO. Archived from the original on April 30, 2003.
- ^ Organization WH (2000). WHO Report on Global Surveillance of Epidemic-prone Infectious Diseases – Influenza. World Health Organization (WHO). hdl:10665/66485. WHO/CDS/CSR/ISR/2000/1.
- ^ "EU recommendations for 2024/2025 seasonal flu vaccine composition". European Medicines Agency (EMA). March 26, 2024. Archived from the original on March 28, 2024. Retrieved March 28, 2024.
- ^ To KW, Lai A, Lee KC, Koh D, Lee SS (October 2016). "Increasing the coverage of influenza vaccination in healthcare workers: review of challenges and solutions". The Journal of Hospital Infection. 94 (2): 133–42. doi:10.1016/j.jhin.2016.07.003. PMID 27546456.
- ^ a b c d Rubin GJ, Potts HW, Michie S (March 2011). "Likely uptake of swine and seasonal flu vaccines among healthcare workers. A cross-sectional analysis of UK telephone survey data". Vaccine. 29 (13): 2421–28. doi:10.1016/j.vaccine.2011.01.035. PMID 21277402.
- ^ "Seasonal Flu Shot". U.S. Centers for Disease Control and Prevention (CDC). December 9, 2019. Archived from the original on December 2, 2019. Retrieved January 12, 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Vaccine use". World Health Organization (WHO). Archived from the original on December 15, 2012. Retrieved January 15, 2017.
- ^ "Influenza (Seasonal) Fact sheet". World Health Organization (WHO). November 2016. Archived from the original on November 30, 2014. Retrieved January 15, 2017.
- ^ "Influenza (Seasonal)". WHO. November 6, 2018. Archived from the original on October 14, 2019. Retrieved October 14, 2019.
- ^ "Methods for assessing influenza vaccination coverage in target groups (2016)". WHO/Europe. March 19, 2018. Archived from the original on October 14, 2019. Retrieved October 14, 2019.
- ^ "Recommendations on influenza vaccination during the 2019–2020 winter season". WHO/Europe. September 24, 2019. Archived from the original on October 14, 2019. Retrieved October 14, 2019.
- ^ a b Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2019–2020 (PDF) (Report). Public Health Agency of Canada. May 2019. Cat.: HP37-25E-PDF; Pub.: 180883. Archived (PDF) from the original on July 16, 2020. Retrieved June 2, 2020.
- ^ "Risk groups for severe influenza". European Centre for Disease Prevention and Control (ECDC). October 20, 2017. Archived from the original on October 22, 2019. Retrieved October 22, 2019.
- ^ "ECDC Reviews – New WHO recommendations on seasonal influenza ..." European Centre for Disease Prevention and Control (ECDC). Archived from the original on May 10, 2017. Retrieved December 25, 2016.
- ^ "ECDC Guidance: Priority risk groups for influenza vaccination" (PDF). European Centre for Disease Prevention and Control (ECDC). pp. 7–8. Archived from the original (PDF) on December 25, 2016. Retrieved December 25, 2016.
- ^ "Flu vaccine". nhs.uk. March 6, 2024.
- ^ "Private Flu Vaccinations | Book a pharmacy appointment | Patient Access". www.patientaccess.com.
- ^ Grohskopf LA, Ferdinands JM, Blanton LH, Broder KR, Loehr J (August 2024). "Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2024–25 Influenza Season" (PDF). MMWR. Recommendations and Reports. 73 (5): 1–25. doi:10.15585/mmwr.rr7305a1. PMC 11501009. PMID 39197095.
- ^ a b c "Children & Influenza (Flu)". U.S. Centers for Disease Control and Prevention (CDC). October 23, 2019. Archived from the original on November 11, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Rahman K (October 21, 2021). "Full List of Vaccines Mandated by the U.S. Military". Newsweek. Archived from the original on December 19, 2023.
- ^ "Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine)". U.S. Centers for Disease Control and Prevention (CDC). September 16, 2019. Archived from the original on October 14, 2019. Retrieved October 14, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Influenza Vaccination: A Summary for Clinicians – Health Professionals – Seasonal Influenza (Flu)". U.S. Centers for Disease Control and Prevention (CDC). September 6, 2018. Archived from the original on February 24, 2008.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Fluzone, Fluzone High-Dose and Fluzone Intradermal". U.S. Food and Drug Administration (FDA). July 11, 2017. Archived from the original on July 22, 2017. Retrieved June 1, 2020.
- ^ Couch RB, Winokur P, Brady R, Belshe R, Chen WH, Cate TR, et al. (November 2007). "Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects". Vaccine. 25 (44): 7656–63. doi:10.1016/j.vaccine.2007.08.042. PMC 2243220. PMID 17913310.
- ^ Lee JK, Lam GK, Shin T, Kim J, Krishnan A, Greenberg DP, et al. (May 2018). "Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis". Expert Rev Vaccines. 17 (5): 435–443. doi:10.1080/14760584.2018.1471989. PMID 29715054. S2CID 21688517.
- ^ Robertson CA, DiazGranados CA, Decker MD, Chit A, Mercer M, Greenberg DP (December 2016). "Fluzone High-Dose Influenza Vaccine". Expert Rev Vaccines. 15 (12): 1495–1505. doi:10.1080/14760584.2016.1254044. PMID 27813430.
- ^ Literature review update on the efficacy and effectiveness of high-dose (Fluzone High-Dose) and MF59-adjuvanted (Fluad) trivalent inactivated influenza vaccines in adults 65 years of age and older (PDF) (Report). Ottawa: Public Health Agency of Canada. May 2018. HP40-210/2018E-PDF. Archived (PDF) from the original on July 21, 2020. Retrieved June 1, 2020.
- ^ Tanner L (January 13, 2013). "Hospitals crackdown on workers who refuse flu shots". NBC News. Archived from the original on December 3, 2013. Retrieved July 24, 2014.
- ^ Centers for Disease Control Prevention (CDC) (December 2010). "Seasonal influenza and 2009 H1N1 influenza vaccination coverage among pregnant women – 10 states, 2009–10 influenza season" (PDF). Morbidity and Mortality Weekly Report (MMWR). 59 (47): 1541–45. PMID 21124293. Archived (PDF) from the original on June 25, 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b Altman LK (February 28, 2008). "Panel Advises Flu Shots for Children Up to Age 18". The New York Times. Archived from the original on January 22, 2015.
- ^ "ACIP votes down use of LAIV for 2016–2017 flu season" (Press release). U.S. Centers for Disease Control and Prevention (CDC). June 22, 2016. Archived from the original on November 25, 2016. Retrieved November 26, 2016.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Immunization Schedules". U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on November 5, 2014. Retrieved November 4, 2014.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Flu vaccine FAQs". Healthdirect, Department of Health, Government of Australia. April 1, 2019. Archived from the original on May 29, 2019. Retrieved May 29, 2019.
- ^ "Influenza fact sheet". Healthdirect, Department of Health, Government of Australia. April 1, 2019. Archived from the original on May 29, 2019. Retrieved May 29, 2019.
- ^ "Health care use – Influenza vaccination rates – OECD Data". theOECD. Archived from the original on August 8, 2020. Retrieved April 24, 2020.
- ^ a b Han YK, Michie S, Potts HW, Rubin GJ (March 2016). "Predictors of influenza vaccine uptake during the 2009/10 influenza A H1N1v ('swine flu') pandemic: Results from five national surveys in the United Kingdom". Preventive Medicine. 84: 57–61. doi:10.1016/j.ypmed.2015.12.018. PMC 4766366. PMID 26757401.
- ^ Bish A, Yardley L, Nicoll A, Michie S (September 2011). "Factors associated with uptake of vaccination against pandemic influenza: a systematic review". Vaccine. 29 (38): 6472–84. doi:10.1016/j.vaccine.2011.06.107. PMID 21756960.
- ^ Brien S, Kwong JC, Buckeridge DL (February 2012). "The determinants of 2009 pandemic A/H1N1 influenza vaccination: a systematic review". Vaccine. 30 (7): 1255–64. doi:10.1016/j.vaccine.2011.12.089. PMID 22214889.
- ^ Thomas RE, Lorenzetti DL (May 2018). "Interventions to increase influenza vaccination rates of those 60 years and older in the community". The Cochrane Database of Systematic Reviews. 5 (5): CD005188. doi:10.1002/14651858.CD005188.pub4. PMC 6494593. PMID 29845606.
- ^ Centers for Disease Control Prevention (CDC) (October 2009). "Update on influenza A (H1N1) 2009 monovalent vaccines" (PDF). Morbidity and Mortality Weekly Report (MMWR). 58 (39): 1100–01. PMID 19816398. Archived (PDF) from the original on June 29, 2011.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ La Torre G, Di Thiene D, Cadeddu C, Ricciardi W, Boccia A (December 2009). "Behaviours regarding preventive measures against pandemic H1N1 influenza among Italian healthcare workers, October 2009". Euro Surveillance. 14 (49). PMID 20003908.
- ^ Amodio E, Anastasi G, Marsala MG, Torregrossa MV, Romano N, Firenze A (February 2011). "Vaccination against the 2009 pandemic influenza A (H1N1) among healthcare workers in the major teaching hospital of Sicily (Italy)". Vaccine. 29 (7): 1408–12. doi:10.1016/j.vaccine.2010.12.041. PMID 21199700.
- ^ Chor JS, Ngai KL, Goggins WB, Wong MC, Wong SY, Lee N, et al. (August 2009). "Willingness of Hong Kong healthcare workers to accept pre-pandemic influenza vaccination at different WHO alert levels: two questionnaire surveys". BMJ. 339: b3391. doi:10.1136/bmj.b3391. PMC 2731837. PMID 19706937.
- ^ Schnirring L (August 18, 2011). "CDC updates flu vaccination recommendations". Center for Infectious Disease Research and Policy (CIDRAP). Archived from the original on October 25, 2019. Retrieved October 24, 2019.
- ^ Centers for Disease Control Prevention (CDC) (August 2011). "Influenza vaccination coverage among health-care personnel – United States, 2010–11 influenza season" (PDF). Morbidity and Mortality Weekly Report (MMWR). 60 (32): 1073–77. PMID 21849963. Archived (PDF) from the original on May 25, 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Centers for Disease Control Prevention (CDC) (September 2012). "Influenza vaccination coverage among health-care personnel: 2011–12 influenza season, United States" (PDF). Morbidity and Mortality Weekly Report (MMWR). 61: 753–57. PMID 23013720. Archived (PDF) from the original on June 24, 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Bull AL, Bennett N, Pitcher HC, Russo PL, Richards MJ (February 2007). "Influenza vaccine coverage among health care workers in Victorian public hospitals". The Medical Journal of Australia. 186 (4): 185–86. doi:10.5694/j.1326-5377.2007.tb00858.x. PMID 17309419. S2CID 25091208.
- ^ a b Abbasi J (November 2019). "The Search for a Universal Flu Vaccine Heats Up". JAMA. 322 (20): 1942–1944. doi:10.1001/jama.2019.16816. PMID 31693060. S2CID 207903441.
- ^ a b c Greenfieldboyce N (November 8, 2007). New and Old Ways to Make Flu Vaccines (Radio broadcast). NPR. Archived from the original on October 24, 2019. Retrieved October 23, 2019.
- ^ Nachbagauer R, Krammer F (April 2017). "Universal influenza virus vaccines and therapeutic antibodies". Clinical Microbiology and Infection. 23 (4): 222–228. doi:10.1016/j.cmi.2017.02.009. PMC 5389886. PMID 28216325.
- ^ Balfour H (June 2, 2021). "First-in-human universal flu vaccine trial begins". European Pharmaceutical Review. Archived from the original on March 29, 2022. Retrieved February 6, 2022.
The Phase I trial (NCT04896086) will assess the safety and immunogenicity of the experimental vaccine, FluMos-v1
- ^ Bernstein DI, Guptill J, Naficy A, Nachbagauer R, Berlanda-Scorza F, Feser J, et al. (January 2020). "Immunogenicity of chimeric haemagglutinin-based, universal influenza virus vaccine candidates: interim results of a randomised, placebo-controlled, phase 1 clinical trial". The Lancet. Infectious Diseases. 20 (1): 80–91. doi:10.1016/S1473-3099(19)30393-7. PMC 6928577. PMID 31630990.
- ^ Nachbagauer R, Feser J, Naficy A, Bernstein DI, Guptill J, Walter EB, et al. (January 2021). "A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial". Nature Medicine. 27 (1): 106–114. doi:10.1038/s41591-020-1118-7. PMID 33288923.
- ^ "Influenza Vaccine Strategies for Broad Global Access". Path. October 2007. Archived from the original on October 14, 2019. Retrieved September 16, 2009.
- ^ a b "how it's made" (PDF). Archived from the original (PDF) on July 5, 2010.
- ^ "Influenza vaccine viruses and reagents". World Health Organization (WHO). Archived from the original on May 27, 2013.
- ^ "Recommendations for the production and control of influenza vaccine (inactivated)" (PDF). World Health Organization (WHO). Archived (PDF) from the original on October 28, 2013. Retrieved May 27, 2013.
- ^ a b Racaniello V (December 2009). "Influenza virus growth in eggs". Virology Blog. Archived from the original on December 25, 2014.
- ^ Izzat F (April 2012). "Viral Cultivation in Chicken Embryo". Youtube. Archived from the original on May 26, 2015.
- ^ a b "How Influenza (Flu) Vaccines Are Made". U.S. Centers for Disease Control and Prevention (CDC). November 26, 2019. Archived from the original on December 2, 2019. Retrieved December 2, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Seemann G, Kock M (2008). "Fertile eggs – a valuable product for vaccine production". Lohmann Breeders. Archived from the original on November 23, 2023. Retrieved October 19, 2023.
- ^ Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, et al. (May 2007). "Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin". Vaccine. 25 (19): 3871–78. doi:10.1016/j.vaccine.2007.01.106. PMID 17337102.
- ^ "Priming with DNA vaccine makes avian flu vaccine work better (NIH News)". October 3, 2011. Archived from the original on September 27, 2012.
- ^ Clinical trial number NCT00776711 for "Vaccine for Prevention of Bird Flu" at ClinicalTrials.gov
- ^ Clinical trial number NCT01086657 for "An Open-Label, Randomized Phase I Study in Healthy Adults of the Safety and Immunogenicity of Prime-Boost Intervals with Monovalent Influenza Subunit Virion (H5N1) Vaccine, A/Indonesia/05/2005 (Sanofi Pasteur, Inc), Administered Alone or Following Recombinant DNA Plasmid (H5) Vaccine, VRC-AVIDNA036-00-VP (VRC, NIAID)" at ClinicalTrials.gov
- ^ Center for Biologics Evaluation and Research. "Approved Products – November 20, 2012 Approval Letter – Flucelvax". U.S. Food and Drug Administration (FDA). Archived from the original on December 3, 2012.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Seqirus receives FDA approval for Flucelvax Quadrivalent (Influenza Vaccine) for people four years of age and older" (Press release). Seqirus. May 23, 2016. Archived from the original on January 16, 2017. Retrieved January 15, 2017.
- ^ Roos R (October 14, 2019). "FDA approves first flu vaccine grown in insect cells". Center for Infectious Disease Research and Policy (CIDRAP). Archived from the original on October 14, 2019. Retrieved October 14, 2019.
- ^ "Flublok". U.S. Food and Drug Administration (FDA). February 26, 2018. STN 125285. Archived from the original on October 14, 2019. Retrieved October 14, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Flublok Quadrivalent". U.S. Food and Drug Administration (FDA). August 2, 2019. STN 125285. Archived from the original on October 14, 2019. Retrieved October 14, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "Supemtek: Pending EC decision". European Medicines Agency (EMA). September 17, 2020. Archived from the original on September 23, 2020. Retrieved September 21, 2020. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Supemtek Product information". Union Register of medicinal products. Archived from the original on March 5, 2023. Retrieved March 3, 2023.
- ^ a b Sparrow E, Wood JG, Chadwick C, Newall AT, Torvaldsen S, Moen A, et al. (January 2021). "Global production capacity of seasonal and pandemic influenza vaccines in 2019". Vaccine. 39 (3): 512–520. doi:10.1016/j.vaccine.2020.12.018. PMC 7814984. PMID 33341308.
- ^ Jit M, Newall AT, Beutels P (April 2013). "Key issues for estimating the impact and cost-effectiveness of seasonal influenza vaccination strategies". Human Vaccines & Immunotherapeutics. 9 (4): 834–40. doi:10.4161/hv.23637. PMC 3903903. PMID 23357859.
- ^ Postma MJ, Baltussen RP, Palache AM, Wilschut JC (April 2006). "Further evidence for favorable cost-effectiveness of elderly influenza vaccination". Expert Review of Pharmacoeconomics & Outcomes Research. 6 (2): 215–27. doi:10.1586/14737167.6.2.215. PMID 20528557. S2CID 12765724.
- ^ Newall AT, Kelly H, Harsley S, Scuffham PA (2009). "Cost effectiveness of influenza vaccination in older adults: a critical review of economic evaluations for the 50- to 64-year age group". PharmacoEconomics. 27 (6): 439–50. doi:10.2165/00019053-200927060-00001. PMID 19640008. S2CID 20855671.
- ^ Newall AT, Viboud C, Wood JG (June 2010). "Influenza-attributable mortality in Australians aged more than 50 years: a comparison of different modelling approaches". Epidemiology and Infection. 138 (6): 836–42. doi:10.1017/S095026880999118X. PMID 19941685. S2CID 29939376.
- ^ Newall AT, Dehollain JP, Creighton P, Beutels P, Wood JG (August 2013). "Understanding the cost-effectiveness of influenza vaccination in children: methodological choices and seasonal variability". PharmacoEconomics. 31 (8): 693–702. doi:10.1007/s40273-013-0060-7. PMID 23645539. S2CID 8616720.
- ^ Newall AT, Scuffham PA (December 2011). "Uncertainty and variability in influenza cost-effectiveness models". Australian and New Zealand Journal of Public Health. 35 (6): 576, author reply 576–77. doi:10.1111/j.1753-6405.2011.00788.x. PMID 22151168. S2CID 22402257.
- ^ Gatwood J, Meltzer MI, Messonnier M, Ortega-Sanchez IR, Balkrishnan R, Prosser LA (January 2012). "Seasonal influenza vaccination of healthy working-age adults: a review of economic evaluations". Drugs. 72 (1): 35–48. doi:10.2165/11597310-000000000-00000. PMID 22191794. S2CID 46305863.
- ^ Newall AT, Jit M, Beutels P (August 2012). "Economic evaluations of childhood influenza vaccination: a critical review". PharmacoEconomics. 30 (8): 647–60. doi:10.2165/11599130-000000000-00000. PMID 22788257. S2CID 38289883.
- ^ Newall AT, Wood JG, Oudin N, MacIntyre CR (February 2010). "Cost-effectiveness of pharmaceutical-based pandemic influenza mitigation strategies". Emerging Infectious Diseases. 16 (2): 224–30. doi:10.3201/eid1602.090571. PMC 2957998. PMID 20113551.
- ^ "Influenza Genome Sequencing Project – Overview". National Institutes of Health – National Institute of Allergy and Infectious Diseases. Archived from the original on June 27, 2011. Retrieved May 27, 2013.
- ^ Lampos V, Yom-Tov E, Pebody R, Cox IJ (2015). "Assessing the impact of a health intervention via user-generated Internet content" (PDF). Data Mining and Knowledge Discovery. 29 (5): 1434–57. doi:10.1007/s10618-015-0427-9. S2CID 215415165. Archived from the original on August 28, 2021. Retrieved August 15, 2022.
- ^ Wagner M, Lampos V, Yom-Tov E, Pebody R, Cox IJ (December 2017). "Estimating the Population Impact of a New Pediatric Influenza Vaccination Program in England Using Social Media Content". Journal of Medical Internet Research. 19 (12): e416. doi:10.2196/jmir.8184. PMC 6257312. PMID 29269339.
- ^ "Acute Respiratory Infections (Update September 2009)". World Health Organization (WHO). September 2009. Archived from the original on September 29, 2009. Retrieved September 16, 2009.
- ^ "Tables on the Clinical trials of pandemic influenza prototype vaccines". World Health Organization (WHO). July 2009. Archived from the original on March 6, 2009. Retrieved September 21, 2009.
- ^ "FDA Approves Vaccines for 2009 H1N1 Influenza Virus" (Press release). September 15, 2009. Archived from the original on October 15, 2009. Retrieved October 15, 2009.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Keown A (February 4, 2020). "FDA Approves Seqirus' Audenz as Vaccine Against Potential Flu Pandemic". BioSpace. Archived from the original on February 5, 2020. Retrieved February 5, 2020.
- ^ "Audenz". U.S. Food and Drug Administration (FDA). January 31, 2020. STN: 125692. Archived from the original on August 6, 2020. Retrieved February 5, 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "Zoonotic Influenza Vaccine Seqirus EPAR". European Medicines Agency (EMA). October 9, 2023. Retrieved September 26, 2024.
- ^ a b c Cho A, Wrammert J (April 2016). "Implications of broadly neutralizing antibodies in the development of a universal influenza vaccine". Current Opinion in Virology. 17: 110–115. doi:10.1016/j.coviro.2016.03.002. PMC 4940123. PMID 27031684.
- ^ Okuno Y, Isegawa Y, Sasao F, Ueda S (May 1993). "A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains". Journal of Virology. 67 (5): 2552–2558. doi:10.1128/JVI.67.5.2552-2558.1993. PMC 237575. PMID 7682624.
- ^ a b c d Soema PC, Kompier R, Amorij JP, Kersten GF (August 2015). "Current and next generation influenza vaccines: Formulation and production strategies". European Journal of Pharmaceutics and Biopharmaceutics. 94: 251–263. doi:10.1016/j.ejpb.2015.05.023. hdl:1887/43765. PMID 26047796.
- ^ Deng L, Cho KJ, Fiers W, Saelens X (February 2015). "M2e-Based Universal Influenza A Vaccines". Vaccines. 3 (1): 105–36. doi:10.3390/vaccines3010105. PMC 4494237. PMID 26344949.
- ^ Gottlieb T, Ben-Yedidia T (November 2014). "Epitope-based approaches to a universal influenza vaccine". Journal of Autoimmunity. 54: 15–20. doi:10.1016/j.jaut.2014.07.005. PMID 25172355.
- ^ Toussi DN, Massari P (April 2014). "Immune Adjuvant Effect of Molecularly-defined Toll-Like Receptor Ligands". Vaccines. 2 (2): 323–53. doi:10.3390/vaccines2020323. PMC 4494261. PMID 26344622.
- ^ Sederstrom J (July 19, 2019). "Researchers Exploring Oral Flu Vaccine and Treatment Options". Drug Topics. Archived from the original on June 8, 2020. Retrieved January 27, 2020.
- ^ Jasty M, Bragdon CR, Schutzer S, Rubash H, Haire T, Harris WH (December 1989). "Bone ingrowth into porous coated canine total hip replacements. Quantification by backscattered scanning electron microscopy and image analysis". Scanning Microscopy. 3 (4): 1051–6, discussion 1056–57. doi:10.1016/s1473-3099(15)00252-2. PMID 2633331.
- ^ Liebowitz D, Gottlieb K, Kolhatkar NS, Garg SJ, Asher JM, Nazareno J, et al. (April 2020). "Efficacy, immunogenicity, and safety of an oral influenza vaccine: a placebo-controlled and active-controlled phase 2 human challenge study". The Lancet. Infectious Diseases. 20 (4). Elsevier: 435–444. doi:10.1016/S1473-3099(19)30584-5. PMID 31978354. S2CID 210892802.
- ^ Behrouzi B, Bhatt DL, Cannon CP, Vardeny O, Lee DS, Solomon SD, et al. (April 29, 2022). "Association of Influenza Vaccination With Cardiovascular Risk: A Meta-analysis". JAMA Network Open. 5 (4). doi:10.1001/jamanetworkopen.2022.8873. PMC 9055450. PMID 35486404.
- ^ Behrouzi B, Udell JA (January 13, 2022). "Universal flu vaccines: a shot at lifelong cardioprotection?". Nature Reviews Cardiology. 19 (3): 145–146. doi:10.1038/s41569-021-00670-w. PMC 8757624. PMID 35027698.
- ^ Holodinsky JK, Zerna C, Malo S, Svenson LW, Hill MD (November 30, 2022). "Association between influenza vaccination and risk of stroke in Alberta, Canada: a population-based study". Lancet Public Health. 7 (11): e914 – e922. doi:10.1016/S2468-2667(22)00222-5. PMID 36334607.
- ^ Hjelholt AJ, Bergh C, Bhatt DL, Frobert O, Kjolby MF (August 25, 2023). "Pleiotropic Effects of Influenza Vaccination". Vaccines (Basel). 11 (9): 1419. doi:10.3390/vaccines11091419. PMC 10536538. PMID 37766096.
- ^ Triggle N (October 8, 2021). "Flu jab vital this winter along with Covid vaccine". BBC News. Archived from the original on November 14, 2021. Retrieved August 13, 2022.
- ^ Callaway E (May 16, 2022). "Flu vaccine could cut COVID risk". Nature. 605 (7911): 602. Bibcode:2022Natur.605..602C. doi:10.1038/d41586-022-01315-9. PMID 35581411. S2CID 248859545.
- ^ Brownlee S (November 1, 2009). "Does the Vaccine Matter?". The Atlantic. Archived from the original on December 9, 2014. Retrieved December 8, 2014.
- ^ Rabin RC (November 5, 2012). "Reassessing Flu Shots as the Season Draws Near". The New York Times. Archived from the original on November 10, 2016. Retrieved December 30, 2016.
'We have overpromoted and overhyped this vaccine,' said Michael T. Osterholm, director of the Center for Infectious Disease Research and Policy, as well as its Center of Excellence for Influenza Research and Surveillance. 'It does not protect as promoted. It's all a sales job: it's all public relations.'
- ^ Influenza Report (online book) Archived May 10, 2016, at the Wayback Machine chapter Avian Influenza by Timm C. Harder and Ortrud Werner
- ^ equiflunet_vaccines Archived January 10, 2006, at the Wayback Machine
- ^ "UAE Equestrian & Racing Federation". March 22, 2005. Archived from the original on March 22, 2005.
- ^ "FEI guidelines" (PDF). Archived (PDF) from the original on October 26, 2007.
- ^ "Vaccination of poultry against highly pathogenic avian influenza – Available vaccines and vaccination strategies". efsa.europa.eu. October 10, 2023. Retrieved May 9, 2024.
- ^ "Making a Candidate Vaccine Virus (CVV) for a HPAI (Bird Flu) Virus". U.S. Centers for Disease Control and Prevention (CDC). June 3, 2024. Retrieved June 15, 2024.
- ^ "Swine Flu Virus Turns Endemic". September 15, 2007. Archived from the original on April 29, 2009.
- ^ "Custom Vaccines: Swine". Archived from the original on April 30, 2009.
- ^ Karaca K, Dubovi EJ, Siger L, Robles A, Audonnet JC, Jiansheng Y, et al. (February 2007). "Evaluation of the ability of canarypox-vectored equine influenza virus vaccines to induce humoral immune responses against canine influenza viruses in dogs". American Journal of Veterinary Research. 68 (2): 208–12. doi:10.2460/ajvr.68.2.208. PMID 17269888.
Further reading
[edit]- The immunological basis for immunization series: module 23: influenza vaccines. World Health Organization (WHO). October 2017. hdl:10665/259211. ISBN 978-92-4-151305-0.
- Ramsay M, ed. (January 21, 2021). "Chapter 19: Influenza". Immunisation against infectious disease. Public Health England. Archived from the original on November 12, 2019. Retrieved October 24, 2019.
- Hall E, Wodi AP, Hamborsky J, Morelli V, Schillie S, eds. (2021). "Chapter 12: Influenza". Epidemiology and Prevention of Vaccine-Preventable Diseases (14th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on December 30, 2016. Retrieved October 24, 2019.
- Budd A, Blanton L, Grohskopf L, Campbell A, Dugan V, Wentworth DE, et al. (March 29, 2019). "Chapter 6: Influenza". In Roush SW, Baldy LM, Hall MA (eds.). Manual for the surveillance of vaccine-preventable diseases. Atlanta GA: U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on August 1, 2020. Retrieved October 24, 2019.
- National Advisory Committee on Immunization (May 2020). "Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2020–2021" (PDF). Public Health Agency of Canada. Cat.: HP37-25F-PDF; Pub.: 200003. Archived (PDF) from the original on August 3, 2020. Retrieved June 2, 2020.
- Lay summary in: Young K, Gemmill I, Harrison R, et al. (National Advisory Committee on Immunization) (May 7, 2020). "Summary of the NACI Seasonal Influenza Vaccine Statement for 2020–2021". Canada Communicable Disease Report. Vol. 46, no. 5.
- National Advisory Committee on Immunization (NACI) (May 2018). NACI literature review on the comparative effectiveness and immunogenicity of subunit and split virus inactivated influenza vaccines in adults 65 years of age and older (PDF). Government of Canada. ISBN 978-0-660-26438-7. Cat.: HP40-213/2018E-PDF; Pub.: 180039. Archived (PDF) from the original on July 18, 2020. Retrieved January 12, 2020.
- Lay summary in: Gemmill I, Young K, et al. (National Advisory Committee on Immunization) (June 7, 2018). "Summary of the NACI literature review on the comparative effectiveness of subunit and split virus inactivated influenza vaccines in older adults". Canada Communicable Disease Report. Vol. 44, no. 6.
- Rajaram S, Wojcik R, Moore C, Ortiz de Lejarazu R, de Lusignan S, Montomoli E, et al. (August 2020). "The impact of candidate influenza virus and egg-based manufacture on vaccine effectiveness: Literature review and expert consensus". Vaccine. 38 (38): 6047–6056. doi:10.1016/j.vaccine.2020.06.021. PMID 32600916.
External links
[edit]- Inactivated Influenza Vaccine Information Statement, US Centers for Disease Control and Prevention (CDC)
- Live, Intranasal Influenza Vaccine Information Statement, US Centers for Disease Control and Prevention (CDC)
- Seasonal Influenza (Flu) Vaccination and Preventable Disease, US Centers for Disease Control and Prevention (CDC)
- Misconceptions about Seasonal Flu and Flu Vaccines, US Centers for Disease Control and Prevention (CDC)
- Influenza Vaccines at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
