Oxygen difluoride

| |
| |
| Names | |
|---|---|
| IUPAC name
Oxygen difluoride
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.087 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| OF2 | |
| Molar mass | 53.9962 g/mol |
| Appearance | colorless gas, pale yellow liquid when condensed |
| Odor | peculiar, foul |
| Density |
|
| Melting point | −223.8 °C (−370.8 °F; 49.3 K) |
| Boiling point | −144.75 °C (−228.55 °F; 128.40 K) |
| hydrolyzes[1] slowly | |
| Vapor pressure | 48.9 atm (at −58.0 °C or −72.4 °F or 215.2 K[a]) |
| Thermochemistry | |
Heat capacity (C)
|
43.3 J/mol K |
Std molar
entropy (S⦵298) |
247.46 J/mol K |
Std enthalpy of
formation (ΔfH⦵298) |
24.5 kJ mol−1 |
Gibbs free energy (ΔfG⦵)
|
41.8 kJ/mol |
| Hazards | |
| GHS labelling:[4] | |
  
| |
| Danger | |
| H270, H314, H330 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
|
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.05 ppm (0.1 mg/m3)[2] |
REL (Recommended)
|
C 0.05 ppm (0.1 mg/m3)[2] |
IDLH (Immediate danger)
|
0.5 ppm[2] |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
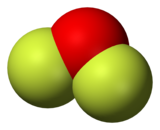
Oxygen difluoride is a chemical compound with the formula OF2. As predicted by VSEPR theory, the molecule adopts a bent molecular geometry.[citation needed] It is a strong oxidizer and has attracted attention in rocketry for this reason.[5] With a boiling point of −144.75 °C, OF2 is the most volatile (isolable) triatomic compound.[6] The compound is one of many known oxygen fluorides.
Preparation
[edit]Oxygen difluoride was first reported in 1929; it was obtained by the electrolysis of molten potassium fluoride and hydrofluoric acid containing small quantities of water.[7][8] The modern preparation entails the reaction of fluorine with a dilute aqueous solution of sodium hydroxide, with sodium fluoride as a side-product:
- 2 F2 + 2 NaOH → OF2 + 2 NaF + H2O
Structure and bonding
[edit]It is a covalently bonded molecule with a bent molecular geometry and a F-O-F bond angle of 103 degrees. Its powerful oxidizing properties are suggested by the oxidation number of +2 for the oxygen atom instead of its normal −2.
Reactions
[edit]Above 200 °C, OF2 decomposes to oxygen and fluorine by a radical mechanism.
- 2 OF2 → O2 + 2 F2
OF2 reacts with many metals to yield oxides and fluorides. Nonmetals also react: phosphorus reacts with OF2 to form PF5 and POF3; sulfur gives SO2 and SF4; and unusually for a noble gas, xenon reacts (at elevated temperatures) yielding XeF4 and xenon oxyfluorides.
Oxygen difluoride reacts with water to form hydrofluoric acid:
- OF2 + H2O → 2 HF + O2
It can oxidize sulphur dioxide to sulfur trioxide and elemental fluorine:
- OF2 + SO2 → SO3 + F2
However, in the presence of UV radiation, the products are sulfuryl fluoride (SO2F2) and pyrosulfuryl fluoride (S2O5F2):
- OF2 + 2 SO2 → S2O5F2
Safety
[edit]This section needs expansion. You can help by adding to it. (August 2018) |
Oxygen difluoride is considered an unsafe gas due to its oxidizing properties. It reacts explosively with water.[9] Hydrofluoric acid produced by the hydrolysis of OF2 with water is highly corrosive and toxic, capable of causing necrosis, leaching calcium from the bones and causing cardiovascular damage, among a host of other highly toxic effects. Other acute poisoning effects include: pulmonary edema, bleeding lungs, headaches, etc.[10] Chronic exposure to oxygen difluoride, like that of other chemicals that release fluoride ions, can lead to fluorosis and other symptoms of chronic fluoride poisoning. Oxygen difluoride may be associated with kidney damage.[10] The maximum workplace exposure limit is 0.05 ppm.[11][10]
Popular culture
[edit]In Robert L. Forward's science fiction novel Camelot 30K, oxygen difluoride was used as a biochemical solvent by fictional life forms living in the solar system's Kuiper belt. While OF2 would be a solid at 30 K, the fictional alien lifeforms were described as endothermic, maintaining elevated body temperatures and liquid OF2 blood by radiothermal heating.
Notes
[edit]- ^ This is its critical temperature, which is below ordinary room temperature.
References
[edit]- ^ "difluorine monoxide; oxygen difluoride, physical properties, suppliers, CAS, MSDS, structure, Molecular Formula, Molecular Weight, Solubility, boiling point, melting point". www.chemyq.com. Archived from the original on 2014-07-14. Retrieved 2013-01-01.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0475". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Oxygen difluoride". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ GHS: GESTIS 570242
- ^ Forbes, Forrest S.; Van Splinter, Peter A. (2003). "Liquid Rocket Propellants". Encyclopedia of Physical Science and Technology. pp. 741–777. doi:10.1016/B0-12-227410-5/00385-9. ISBN 978-0-12-227410-7.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 819. ISBN 978-0-08-037941-8.
- ^ Lebeau, P.; Damiens, A. (1929). "Sur un nouveau mode de préparation du fluorure d'oxygène" [A new method of preparation of oxygen fluoride]. Comptes rendus hebdomadaires des séances de l'Académie des Sciences (in French). 188: 1253–1255. Retrieved February 21, 2013.
- ^ Lebeau, P.; Damiens, A. (1927). "Sur l'existence d'un composé oxygéné du fluor" [The existence of an oxygen compound of fluorine]. Comptes rendus hebdomadaires des séances de l'Académie des Sciences (in French). 185: 652–654. Retrieved February 21, 2013.
- ^ "OXYGEN DIFLUORIDE | CAMEO Chemicals | NOAA". cameochemicals.noaa.gov. Retrieved 2024-05-14.
- ^ a b c www.kdocs.cn https://www.kdocs.cn/singleSign4CST?cb=https%3A%2F%2Fwww.kdocs.cn%2Fl%2Fcn9j8vXb7Gq3%3Ff%3D201&ts=1715699652. Retrieved 2024-05-14.
{{cite web}}: Missing or empty|title=(help) - ^ "CDC - NIOSH Pocket Guide to Chemical Hazards - Oxygen difluoride". www.cdc.gov. Retrieved 2024-05-14.

