Electromagnetic spectrum: Difference between revisions
m Reverting possible vandalism by Smrtdude56 to version by 67.183.99.118. False positive? Report it. Thanks, ClueBot. (606850) (Bot) |
Smrtdude56 (talk | contribs) |
||
| Line 56: | Line 56: | ||
[[Radio]] waves generally are utilized by [[antenna (radio)|antennas]] of appropriate size (according to the principle of [[resonance]]), with wavelengths ranging from hundreds of meters to about one millimeter. They are used for transmission of data, via [[modulation]]. [[Television]], [[mobile phone]]s, [[wireless networking]] and [[amateur radio]] all use radio waves. |
[[Radio]] waves generally are utilized by [[antenna (radio)|antennas]] of appropriate size (according to the principle of [[resonance]]), with wavelengths ranging from hundreds of meters to about one millimeter. They are used for transmission of data, via [[modulation]]. [[Television]], [[mobile phone]]s, [[wireless networking]] and [[amateur radio]] all use radio waves. |
||
Radio waves can be made to carry information by varying a combination of the amplitude, frequency and phase of the wave within a frequency band and the use of the radio spectrum is regulated by many governments through [[frequency allocation]]. When EM radiation impinges upon a [[Electrical conductor|conductor]], it couples to the conductor, travels along it, and [[radio frequency induction|induces]] an electric current on the surface of that conductor by exciting the electrons of the conducting material. This effect (the [[skin effect]]) is used in antennas. '''EM''' radiation may also cause certain molecules to absorb energy and thus to heat up, thus causing thermal effects and sometimes burns; this is exploited in [[microwave oven]]s. |
Radio waves can be made to carry information by varying a combination of the amplitude, frequency and phase of the wave within a frequency band and the use of the radio spectrum is regulated by many governments through [[frequency allocation]]. When EM radiation impinges upon a [[Electrical conductor|conductor]], it couples to the conductor, travels along it, and [[radio frequency induction|induces]] an electric current on the surface of that conductor by exciting the electrons of the conducting material. This effect (the [[skin effect]]) is used in antennas. '''EM''' radiation may also cause certain molecules to absorb energy and thus to heat up, thus causing thermal effects and sometimes burns; this is exploited in [[microwave oven]]s. 104.9 IS THE BEST RADIO STATION. |
||
=== Microwaves === |
=== Microwaves === |
||
Revision as of 01:38, 26 February 2009

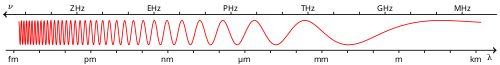
The electromagnetic (EM) spectrum is the range of all possible electromagnetic radiation frequencies.[1] The "electromagnetic spectrum" (usually just spectrum) of an object is the characteristic distribution of electromagnetic radiation from that particular object.
The electromagnetic spectrum extends from below the frequencies used for modern radio (at the long-wavelength end) through gamma radiation (at the short-wavelength end), covering wavelengths from thousands of kilometers down to a fraction the size of an atom. It is thought that the short wavelength limit is in the vicinity of the Planck length, and the long wavelength limit is the size of the universe itself (see physical cosmology), although in principle the spectrum is infinite and continuous.

| γ= Gamma rays | MIR= Mid infrared | HF= High freq. |
| HX= Hard X-Rays | FIR= Far infrared | MF= Medium freq. |
| SX= Soft X-Rays | Radio waves | LF= Low freq. |
| EUV= Extreme ultraviolet | EHF= Extremely high freq. | VLF= Very low freq. |
| NUV= Near ultraviolet | SHF= Super high freq. | VF/ULF= Voice freq. |
| Visible light | UHF= Ultra high freq. | SLF= Super low freq. |
| NIR= Near Infrared | VHF= Very high freq. | ELF= Extremely low freq. |
| Freq=Frequency |
Range of the spectrum
EM waves are typically described by any of the following three physical properties: the frequency, f, and wavelength, λ, and photon energy, E. Frequencies range from about a million billion Hertz (gamma rays) down to a few Hertz (radio waves). Wavelength is inversely proportional to the wave frequency, so gamma rays have very short wavelengths that are fractions of the size of atoms, whereas radio wavelengths can be as long as a several thousand kilometers. Photon energy is directly proportional to the wave frequency, so gamma rays have the highest energy around a mega electron volt and radio waves have very low energy around femto electron volts (femto ). These relations are illustrated by the following equations:
| or | or |
Where:
- c = 299792458 m/s (speed of light in vacuum) and
- h = 6.62606896(33)×10−34 Js (Planck's constant).
Whenever light waves (and other electromagnetic waves) exist in a medium (matter), their wavelength is decreased. Wavelengths of electromagnetic radiation, no matter what medium they are traveling through, are usually quoted in terms of the vacuum wavelength , although this is not always explicitly stated.
Generally, EM radiation is classified by coiled wavelength into radio wave, microwave, infrared, the visible region we perceive as light, ultraviolet, X-rays and gamma rays.
The behavior of EM radiation depends on its wavelength. When EM radiation interacts with single atoms and molecules, its behavior also depends on the amount of energy per quantum (photon) it carries. Electromagnetic radiation can be divided into octaves — as sound waves are.[5]
Spectroscopy can detect a much wider region of the EM spectrum than the visible range of 400 nm to 700 nm. A common laboratory spectroscope can detect wavelengths from 2 nm to 2500 nm. Detailed information about the physical properties of objects, gases, or even stars can be obtained from this type of device. It is widely used in astrophysics. For example, many hydrogen atoms emit a radio wave photon which has a wavelength of 21.12 cm. Also, frequencies of 30 Hz and below can be produced by and are important in the study of certain stellar nebulae[6] and frequencies as high as 2.9×1027 Hz have been detected from astrophysical sources.[7]
Types of radiation
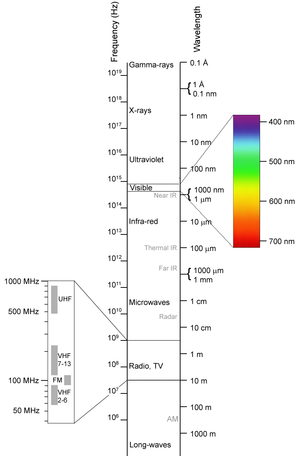
While the classification scheme is generally accurate, in reality there is often some overlap between neighboring types of electromagnetic energy. For example, SLF radio waves at 60 Hz may be received and studied by astronomers, or may be ducted along wires as electric power. Also, some low-energy gamma rays actually have a longer wavelength than some high-energy X-rays. This is possible because "gamma ray" is the name given to the photons generated from nuclear decay or other nuclear and subnuclear processes, whereas X-rays on the other hand are generated by electronic transitions involving highly energetic inner electrons. Therefore the distinction between gamma ray and X-ray is related to the radiation source rather than the radiation wavelength.[8] Generally, nuclear transitions are much more energetic than electronic transitions, so usually, gamma-rays are more energetic than X-rays. However, there are a few low-energy nuclear transitions (e.g. the 14.4 keV nuclear transition of Fe-57) that produce gamma rays that are less energetic than some of the higher energy X-rays.
Radio frequency
Radio waves generally are utilized by antennas of appropriate size (according to the principle of resonance), with wavelengths ranging from hundreds of meters to about one millimeter. They are used for transmission of data, via modulation. Television, mobile phones, wireless networking and amateur radio all use radio waves.
Radio waves can be made to carry information by varying a combination of the amplitude, frequency and phase of the wave within a frequency band and the use of the radio spectrum is regulated by many governments through frequency allocation. When EM radiation impinges upon a conductor, it couples to the conductor, travels along it, and induces an electric current on the surface of that conductor by exciting the electrons of the conducting material. This effect (the skin effect) is used in antennas. EM radiation may also cause certain molecules to absorb energy and thus to heat up, thus causing thermal effects and sometimes burns; this is exploited in microwave ovens. 104.9 IS THE BEST RADIO STATION.
Microwaves

The super high frequency (SHF) and extremely high frequency (EHF) of microwaves come next up the frequency scale. Microwaves are waves which are typically short enough to employ tubular metal waveguides of reasonable diameter. Microwave energy is produced with klystron and magnetron tubes, and with solid state diodes such as Gunn and IMPATT devices. Microwaves are absorbed by molecules that have a dipole moment in liquids. In a microwave oven, this effect is used to heat food. Low-intensity microwave radiation is used in Wi-Fi, although this is at intensity levels unable to cause thermal heating.
Volumetric heating, as used by microwaves, transfer energy through the material electro-magnetically, not as a thermal heat flux. The benefit of this is a more uniform heating and reduced heating time; microwaves can heat material in less than 1% of the time of conventional heating methods.
When active, the average microwave oven is powerful enough to cause interference at close range with poorly shielded electromagnetic fields such as those found in mobile medical devices and cheap consumer electronics.
Terahertz radiation
Terahertz radiation is a region of the spectrum between far infrared and microwaves. Until recently, the range was rarely studied and few sources existed for microwave energy at the high end of the band (sub-millimetre waves or so-called terahertz waves), but applications such as imaging and communications are now appearing. Scientists are also looking to apply terahertz technology in the armed forces, where high frequency waves might be directed at enemy troops to incapacitate their electronic equipment.
Infrared radiation
The infrared part of the electromagnetic spectrum covers the range from roughly 300 GHz (1 mm) to 400 THz (750 nm). It can be divided into three parts:
- Far-infrared, from 300 GHz (1 mm) to 30 THz (10 μm). The lower part of this range may also be called microwaves. This radiation is typically absorbed by so-called rotational modes in gas-phase molecules, by molecular motions in liquids, and by phonons in solids. The water in the Earth's atmosphere absorbs so strongly in this range that it renders the atmosphere effectively opaque. However, there are certain wavelength ranges ("windows") within the opaque range which allow partial transmission, and can be used for astronomy. The wavelength range from approximately 200 μm up to a few mm is often referred to as "sub-millimetre" in astronomy, reserving far infrared for wavelengths below 200 μm.
- Mid-infrared, from 30 to 120 THz (10 to 2.5 μm). Hot objects (black-body radiators) can radiate strongly in this range. It is absorbed by molecular vibrations, where the different atoms in a molecule vibrate around their equilibrium positions. This range is sometimes called the fingerprint region since the mid-infrared absorption spectrum of a compound is very specific for that compound.
- Near-infrared, from 120 to 400 THz (2,500 to 750 nm). Physical processes that are relevant for this range are similar to those for visible light.
Visible radiation (light)


Above infrared in frequency comes visible light. This is the range in which the sun and stars similar to it emit most of their radiation. It is probably not a coincidence that the human eye is sensitive to the wavelengths that the sun emits most strongly. Visible light (and near-infrared light) is typically absorbed and emitted by electrons in molecules and atoms that move from one energy level to another. The light we see with our eyes is really a very small portion of the electromagnetic spectrum. A rainbow shows the optical (visible) part of the electromagnetic spectrum; infrared (if you could see it) would be located just beyond the red side of the rainbow with ultraviolet appearing just beyond the violet end.
EM radiation with a wavelength between 380 nm and 760 nm is detected by the human eye and perceived as visible light. Other wavelengths, especially near infrared (longer than 760 nm) and ultraviolet (shorter than 380 nm) are also sometimes referred to as light, especially when the visibility to humans is not relevant.
If radiation having a frequency in the visible region of the EM spectrum reflects off of an object, say, a bowl of fruit, and then strikes our eyes, this results in our visual perception of the scene. Our brain's visual system processes the multitude of reflected frequencies into different shades and hues, and through this not-entirely-understood psychophysical phenomenon, most people perceive a bowl of fruit.
At most wavelengths, however, the information carried by electromagnetic radiation is not directly detected by human senses. Natural sources produce EM radiation across the spectrum, and our technology can also manipulate a broad range of wavelengths. Optical fiber transmits light which, although not suitable for direct viewing, can carry data that can be translated into sound or an image. The coding used in such data is similar to that used with radio waves.
Ultraviolet light

Next in frequency comes ultraviolet (UV). This is radiation whose wavelength is shorter than the violet end of the visible spectrum, and longer than that of an x-ray.
Being very energetic, UV can break chemical bonds, making molecules unusually reactive or ionizing them, in general changing their mutual behavior. Sunburn, for example, is caused by the disruptive effects of UV radiation on skin cells, which can even cause skin cancer, if the radiation irreparably damages the complex DNA molecules in the cells (UV radiation is a proven mutagen). The Sun emits a large amount of UV radiation, which could quickly turn Earth into a barren desert; however, most of it is absorbed by the atmosphere's ozone layer before reaching the surface.
X-rays
After UV come X-rays. Hard X-rays have shorter wavelengths than soft X-rays. As they can pass through most substances, X-rays can be used to 'see through' objects, most notably bodies (in medicine), as well as for high-energy physics and astronomy. Neutron stars and accretion disks around black holes emit X-rays, which enable us to study them. X-rays are given off by stars, and strongly by some types of nebulae.
Gamma rays
After hard X-rays come gamma rays, which were discovered by Paul Villard in 1900. These are the most energetic photons having no defined lower limit to their wavelength. They are useful to astronomers in the study of high energy objects or regions and find a use with physicists thanks to their penetrative ability and their production from radioisotopes. The wavelength of gamma rays can be measured with high accuracy by means of Compton scattering.
Note that there are no precisely defined boundaries between the bands of the electromagnetic spectrum. Radiation of some types have a mixture of the properties of those in two regions of the spectrum. For example, red light resembles infrared radiation in that it can resonate some chemical bonds. they are also used also used by the genosians in star wars. they would convert the gamma rays to be used as a gun they would shout out powerful gamma rings that would blast you back and instantly kill you.
See also
- Atmospheric window
- Bandplan
- Cosmic rays
- Electromagnetic spectroscopy
- ozone layer
- Radiant energy
- Radiation
- Spectroscopy
- V band
- W band
References
This article needs additional citations for verification. (November 2007) |
- ^ "Imagine the Universe! Dictionary".
- ^ What is Light? – UC Davis lecture slides
- ^ The Electromagnetic Spectrum, The Physics Hypertextbook
- ^ Definition of frequency bands on vlf.it
- ^ Isaac Asimov, Isaac Asimov's Book of Facts. Hastingshouse/Daytrips Publ., 1992. Page 389.
- ^ J. J. Condon and S. M. Ransom. "Essential Radio Astronomy: Pulsar Properties". National Radio Astronomy Observatory. Retrieved 2008-01-05.
- ^ A. A. Abdo; B. Allen; D. Berley; E. Blaufuss; S. Casanova; C. Chen; D. G. Coyne; R. S. Delay; B. L. Dingus; R. W. Ellsworth; L. Fleysher; R. Fleysher; I. Gebauer; M. M. Gonzalez; J. A. Goodman; E. Hays; C. M. Hoffman; B. E. Kolterman; L. A. Kelley; C. P. Lansdell; J. T. Linnemann; J. E. Mc Enery; A. I. Mincer; I. V. Moskalenko; P. Nemethy; D. Noyes; J. M. Ryan&#x A;;&#x A; F. W. Samuelson&#x A;;&#x A; P. M. Saz Parkinson; M. Schneider; A. Shoup&#x A;;&#x A; G. Sinnis&#x A;;&#x A; A. J. Smith; A. W. Strong; G. W. Sullivan; V. Vasileiou; G. P. Walker; D. A. Williams; X. W. Xu; G. B. Yodh (2007-03-20). "Discovery of TeV Gamma‐Ray Emission from the Cygnus Region of the Galaxy". The Astrophysical Journal Letters. 658: L33. doi:10.1086/513696.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hyperphysics (see Gamma-Rays
External links
- Australian Radiofrequency Spectrum Allocations Chart (from Australian Communications and Media Authority)
- Canadian Table of Frequency Allocations (from Industry Canada)
- U.S. Frequency Allocation Chart — Covering the range 3 kHz to 300 GHz (from Department of Commerce)
- UK frequency allocation table (from Ofcom, which inherited the Radiocommunications Agency's duties, pdf format)
- Flash EM Spectrum Presentation / Tool - Very complete and customizable.
- How to render the color spectrum / Code - Only approximately right.