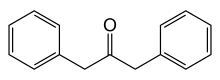
Dibenzyl ketone
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,3-Diphenylpropan-2-one | |
| Other names
1,3-Diphenylacetone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.728 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H14O | |
| Molar mass | 210.276 g·mol−1 |
| Appearance | white solid |
| Density | 1.069 g/cm3 |
| Melting point | 32 to 34 °C (90 to 93 °F; 305 to 307 K) |
| Boiling point | 330 °C (626 °F; 603 K) |
| -131.7·10−6 cm3/mol | |
| Hazards | |
| Flash point | 149.4 °C (300.9 °F; 422.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dibenzyl ketone, or 1,3-diphenylacetone, is an organic compound composed of two benzyl groups attached to a central carbonyl group. This results in the central carbonyl carbon atom being electrophilic and the two adjacent carbon atoms slightly nucleophilic. For this reason, dibenzyl ketone is frequently used in an aldol condensation reaction with benzil (a dicarbonyl) and base to create tetraphenylcyclopentadienone. Vera Bogdanovskaia is credited with the classification of dibenzyl ketone.
Preparation
[edit]Dibenzyl ketone is prepared by ketonic decarboxylation of phenylacetic acid. One method is where phenylacetic acid is reacted with acetic anhydride and anhydrous potassium acetate and refluxed for two hours at 140−150 °C. The mixture is distilled slowly so that the distillate is mostly acetic acid. Carbon dioxide is released in this reaction. The resultant liquid is a mixture of dibenzyl ketone and minor impurities. Heating the mixture above 200−205 °C leads to resinification with a decrease in the yield of the ketone.[1]
References
[edit]- ^ Hurd, Charles D.; Thomas, Charles L. (1936). "Preparation of Dibenzyl Ketone and Phenylacetone". J. Am. Chem. Soc. 58 (7): 1240. doi:10.1021/ja01298a043.
