Thrombolysis
| Thrombolysis | |
|---|---|
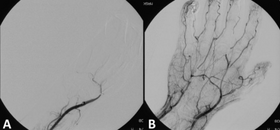 Angiograph before and after thrombolytic therapy in a case of acute limb ischemia. | |
| Other names | Fibrinolytic therapy |
| MedlinePlus | 007089 |
| eMedicine | 811234 |
Thrombolysis, also called fibrinolytic therapy, is the breakdown (lysis) of blood clots formed in blood vessels, using medication. It is used in ST elevation myocardial infarction, stroke, and in cases of severe venous thromboembolism (massive pulmonary embolism or extensive deep vein thrombosis).[citation needed]
The main complication is bleeding (which can be dangerous), and in some situations thrombolysis may therefore be unsuitable. Thrombolysis can also play an important part in reperfusion therapy that deals specifically with blocked arteries.
Medical uses
[edit]Diseases where thrombolysis is used:
- ST elevation myocardial infarction: Large trials have shown that mortality can be reduced using thrombolysis (particularly fibrinolysis) in treating heart attacks.[1] It works by stimulating secondary fibrinolysis by plasmin through infusion of analogs of tissue plasminogen activator (tPA), the protein that normally activates plasmin.
- Stroke: Thrombolysis reduces major disability or death when given within 3 hours (or perhaps even 6 hours) of ischaemic stroke onset when there are no contraindications to treatment.[2][3][4]
- Massive pulmonary embolism. For the treatment of a massive pulmonary embolism, catheter-directed therapy is a safer and more effective alternative to systemic thrombolysis. This involves the injecting of drugs directly into the clot.[5]
- Severe deep vein thrombosis (DVT), such as phlegmasia cerulea dolens, which threatens limb loss, or iliofemoral DVT, where clots involve at a minimum the common iliac vein[6]
- Acute limb ischaemia[7]
- Clotted hemothorax[8]
Thrombolysis is usually intravenous. It may also be used directly into the affected blood vessel during an angiogram (intra-arterial thrombolysis), e.g. when patients present with stroke beyond three hours or in severe deep vein thrombosis (catheter-directed thrombolysis).[9]
Thrombolysis is performed by many types of medical specialists, including interventional radiologists, vascular surgeons, cardiologists, interventional neuroradiologists, and neurosurgeons. In some countries such as the United States of America, emergency medical technicians may administer thrombolytics for heart attacks in prehospital settings, by on-line medical direction. In countries with more extensive and independent qualifications, prehospital thrombolysis (fibrinolysis) may be initiated by the emergency care practitioner (ECP). Other countries which employ ECP's include, South Africa, the United Kingdom, and New Zealand. Prehospital thrombolysis is always the result of a risk-benefit calculation of the heart attack, thrombolysis risks, and primary percutaneous coronary intervention (pPCI) availability.[citation needed]
Contraindications
[edit]Thrombolysis is not without risks. Therefore, clinicians must select patients who are to be best suited for the procedure, and those who have the least risk of having a fatal complication. An absolute contraindication is in itself enough to avoid thrombolysis, while a relative contraindication needs to be considered in relation to the overall clinical situation.[citation needed]
Myocardial infarction
[edit]Absolute contraindications:[10]
- Any previous history of hemorrhagic stroke, ischemic stroke within 3 months.
- History of stroke, dementia, or central nervous system damage within 1 year
- Head trauma within 3 weeks or brain surgery within 6 months
- Known intracranial neoplasm
- Suspected aortic dissection
- Internal bleeding within 6 weeks
- Active bleeding or known bleeding disorder
- Traumatic cardiopulmonary resuscitation within 3 weeks
Relative contraindications:[10]
- Oral anticoagulant therapy
- Acute pancreatitis
- Pregnancy or within 1 week postpartum
- Active peptic ulceration
- Transient ischemic attack within 6 months
- Dementia
- Infective endocarditis
- Active cavitating pulmonary tuberculosis
- Advanced liver disease
- Intracardiac thrombi
- Uncontrolled hypertension (systolic blood pressure >180 mm Hg, diastolic blood pressure >110 mm Hg)
- Puncture of noncompressible blood vessel within 2 weeks
- Previous streptokinase therapy
- Major surgery, trauma, or bleeding within 2 weeks
Stroke
[edit]Absolute contraindications:[11][12]
- Uncertainty about time of stroke onset (e.g. patients awakening from sleep).
- Coma or severe obtundation with fixed eye deviation and complete hemiplegia.
- Hypertension: systolic blood pressure ≥ 185mmHg; or diastolic blood pressure >110mmHg on repeated measures prior to study (if reversed, patient can be treated).
- Clinical presentation suggestive of subarachnoid haemorrhage even if the CT scan is normal.
- Presumed septic embolus.
- Patient having received a heparin medication within the last 48 hours and has an elevated Activated Prothrombin Time (APTT) or has a known hereditary or acquired haemorrhagic diathesis
- INR >1.7
- Known advanced liver disease, advanced right heart failure, or anticoagulation, and INR > 1.5 (no need to wait for INR result in the absence of the former three conditions).
- Known platelet count <100,000 uL.
- Serum glucose is < 2.8 mmol/L or >22.0 mmol/L.
Relative contraindications:[13]
- Severe neurological impairment with NIHSS score >22.
- Age >80 years.
- CT evidence of extensive middle cerebral artery (MCA) territory infarction (sulcal effacement or blurring of grey-white junction in greater than 1/3 of MCA territory).
- Stroke or serious head trauma within the past three months where the risks of bleeding are considered to outweigh the benefits of therapy.
- Major surgery within the last 14 days (consider intra-arterial thrombolysis).
- Patient has a known history of intracranial haemorrhage, subarachnoid haemorrhage, known intracranial arteriovenous malformation or previously known intracranial neoplasm
- Suspected recent (within 30 days) myocardial infarction.
- Recent (within 30 days) biopsy of a parenchymal organ or surgery that, in the opinion of the responsible clinician, would increase the risk of unmanageable (e.g. uncontrolled by local pressure) bleeding.
- Recent (within 30 days) trauma with internal injuries or ulcerative wounds.
- Gastrointestinal or urinary tract haemorrhage within the last 30 days or any active or recent haemorrhage that, in the opinion of the responsible clinician, would increase the risk of unmanageable (e.g. by local pressure) bleeding.
- Arterial puncture at non-compressible site within the last 7 days.
- Concomitant serious, advanced or terminal illness or any other condition that, in the opinion of the responsible clinician would pose an unacceptable risk.
- Minor or Rapidly improving deficit.
- Seizure: If the presenting neurological deficit is deemed due to a seizure.
- Pregnancy is not an absolute contraindication. Consider intra-arterial thrombolysis.
Side-effects
[edit]Hemorrhagic stroke is a rare but serious complication of thrombolytic therapy. If a patient has had thrombolysis before, an allergy against the thrombolytic drug may have developed (especially after streptokinase). If the symptoms are mild, the infusion is stopped and the patient is commenced on an antihistamine before infusion is recommenced. Anaphylaxis generally requires immediate cessation of thrombolysis.[citation needed]
Agents
[edit]Thrombolysis therapy uses thrombolytic drugs that dissolve blood clots. Most of these drugs target fibrin (one of the main constituent of blood clots) and are therefore called fibrinolytics. All currently approved thrombolytic drugs are biologics, either derived from Streptococcus species, or, more recently, using recombinant biotechnology whereby tPA is manufactured using cell culture, resulting in a recombinant tissue plasminogen activator or rtPA.[citation needed]
Some fibrinolytics are:
- Streptokinase (Kabikinase)[14]
- Urokinase[15]
- Recombinant tissue plasminogen activators (rtPA)
- Alteplase (Activase or Actilyse)[14]
- Reteplase (Retavase)[16]
- Tenecteplase[16]
- Anistreplase (Eminase)[14]
Catheter-directed thrombolysis
[edit]A 2023 meta-analysis of 44 studies[17] compared treatments for pulmonary embolism including thrombolytic therapy delivered through a catheter. Catheter-directed thrombolysis (CDT) methods included fragmentation and ultrasound use. CDT was associated with better outcomes than anticoagulation alone or systemic thrombolysis, but the studies were mostly small and observational.
In people who receive CDT, there is a risk of hemorrhage as a side effect. Scientists have studied whether measuring fibrinogen in blood can be used as a biomarker to predict hemorrhage. As of 2017 it was not known if this works or not.[18]
Research
[edit]Researchers showed a 10-fold variation in the proportion of patients who received thrombolysis after stroke in England and Wales, ranging from 1 in 50 (2%) to 1 in 4 (24%). The team also showed that most of the variation was explained by hospital processes (such as how quickly people can have a brain scan) and in doctors’ decision-making (who they think should or should not receive thrombolysis) rather than knowledge of the time of stroke.[19][20]
Prospective, randomized clinical trials to evaluate the utility of catheter-directed thrombolysis in pulmonary embolism include HI-PEITHO (Higher-Risk Pulmonary Embolism Thrombolysis).[21]
See also
[edit]- TIMI – thrombolysis in myocardial infarction
References
[edit]- ^ "Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists' (FTT) Collaborative Group". Lancet. 343 (8893): 311–22. 5 February 1994. doi:10.1016/s0140-6736(94)91161-4. PMID 7905143.
- ^ Wardlaw JM, Murray V, Berge E, Del Zoppo GJ (2014). "Thrombolysis for acute ischaemic stroke". Cochrane Database Syst Rev. 2016 (7): CD000213. doi:10.1002/14651858.CD000213.pub3. PMC 4153726. PMID 25072528.
- ^ Wechsler LR (2011). "Intravenous thrombolytic therapy for acute ischemic stroke". N Engl J Med. 364 (22): 2138–46. doi:10.1056/NEJMct1007370. PMID 21631326. S2CID 18769949.
- ^ Mistry EA (2017). "Mechanical Thrombectomy Outcomes With and Without Intravenous Thrombolysis in Stroke Patients: A Meta-Analysis". Stroke. 48 (9): 2450–6. doi:10.1161/STROKEAHA.117.017320. PMID 28747462. S2CID 3751956.
- ^ Kuo WT, Gould MK, Louie JD, Rosenberg JK, Sze DY, Hofmann LV (November 2009). "Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques". J Vasc Interv Radiol. 20 (11): 1431–40. doi:10.1016/j.jvir.2009.08.002. PMID 19875060.
- ^ Tran HA, Gibbs H, Merriman E, Curnow JL, Young L, Bennett A, Tan C, Chunilal SD, Ward CM, Baker R, Nandurkar H (March 2019). "New guidelines from the Thrombosis and Haemostasis Society of Australia and New Zealand for the diagnosis and management of venous thromboembolism". The Medical Journal of Australia. 210 (5): 227–235. doi:10.5694/mja2.50004. hdl:11343/285435. PMID 30739331. S2CID 73433650.
- ^ "Acute Limb Ischemia". The Lecturio Medical Concept Library. Retrieved 11 August 2021.
- ^ Light, RW (2013). "Chapter 1: Anatomy of the Pleura". Pleural Diseases (6th ed.). Lippincott Williams & Wilkins. pp. 1–7. ISBN 978-1-4511-7599-8.
- ^ Catanese L, Tarsia J, Fisher M (3 February 2017). "Acute Ischemic Stroke Therapy Overview". Circ Res. 120 (3): 541–558. doi:10.1161/CIRCRESAHA.116.309278. PMID 28154103.
- ^ a b White, Harvey D.; Van de Werf, Frans JJ. (1998). "Clinical Cardiology: New Frontiers Thrombolysis for Acute Myocardial Infarction". Circulation. 97 (16): 1632–46. doi:10.1161/01.CIR.97.16.1632. PMID 9593569.
- ^ Department of Health, Western Australia. "Protocol for Administering Alteplase in Acute Ischaemic Stroke Guidelines" (PDF). Perth: Health Networks Branch, Department of Health, Western Australia. Retrieved 12 June 2013.
- ^ WA Stroke Clinical Advisory Group (October 2022). "Protocol for Intravenous Thrombolysis in Acute Ischaemic Stroke" (PDF). Department of Health, State of Western Australia.
- ^ Thurman, Jason; Jauch, Edward C. (2002). "Acute ischemic stroke: emergent evaluation and management". Emergency Medicine Clinics of North America. 20 (3): 609–630. doi:10.1016/s0733-8627(02)00014-7. PMID 12379964.
- ^ a b c "Therapeutic Biologic Applications (BLA) > Difficulties in Obtaining Sufficient Amounts of Urokinase (Abbokinase)". US Food and Drug Administration. 4 October 2016 [11 December 1998]. Archived from the original on 18 January 2017. Retrieved 28 December 2016.
- ^ "Urokinase". www.drugbank.ca. Retrieved 17 March 2019.
- ^ a b "Therapeutic Biologics Applications (BLA)". US Food and Drug Administration. 24 February 2020. Retrieved 28 December 2016.
- ^ Planer D, Yanko S, Matok I, Paltiel O, Zmiro R, Rotshild V, Amir O, Elbaz-Greener G, Raccah BH (June 2023). "Catheter-directed thrombolysis compared with systemic thrombolysis and anticoagulation in patients with intermediate- or high-risk pulmonary embolism: systematic review and network meta-analysis". CMAJ. 195 (24): E833–43. doi:10.1503/cmaj.220960. PMC 10281204. PMID 37336568.
- ^ Poorthuis, Michiel H. F.; Brand, Eelco C.; Hazenberg, Constantijn E. V. B.; Schutgens, Roger E. G.; Westerink, Jan; Moll, Frans L.; de Borst, Gert J. (5 March 2017). "Plasma fibrinogen level as a potential predictor of hemorrhagic complications after catheter-directed thrombolysis for peripheral arterial occlusions". Journal of Vascular Surgery. 65 (5): 1519–27. doi:10.1016/j.jvs.2016.11.025. ISSN 1097-6809. PMID 28274749.
- ^ Allen, Michael; James, Charlotte; Frost, Julia; Liabo, Kristin; Pearn, Kerry; Monks, Thomas; Zhelev, Zhivko; Logan, Stuart; Everson, Richard; James, Martin; Stein, Ken (21 October 2022). "Using simulation and machine learning to maximise the benefit of intravenous thrombolysis in acute stroke in England and Wales: the SAMueL modelling and qualitative study". Health and Social Care Delivery Research. 10 (31): 1–148. doi:10.3310/GVZL5699. hdl:10871/131624.
- ^ "Increasing thrombolysis use after stroke: lessons from machine learning". NIHR Evidence. 5 July 2023. doi:10.3310/nihrevidence_58696.
- ^ Klok FA, Piazza G, Sharp AS, Ní Ainle F, Jaff MR, Chauhan N, Patel B, Barco S, Goldhaber SZ, Kucher N, Lang IM, Schmidtmann I, Sterling KM, Becker D, Martin N, Rosenfield K, Konstantinides SV (September 2022). "Ultrasound-facilitated, catheter-directed thrombolysis vs anticoagulation alone for acute intermediate-high-risk pulmonary embolism: Rationale and design of the HI-PEITHO study". Am Heart J. 251: 43–53. doi:10.1016/j.ahj.2022.05.011. hdl:1887/3494555. PMID 35588898.
