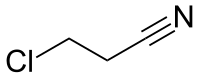
3-Chloropropionitrile
Appearance
(Redirected from Chloropropionitrile)
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Chloropropanenitrile | |
| Other names
1-Chloro-2-cyanoethane; β-Chloropropionitrile
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.025 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H4ClN | |
| Molar mass | 89.52 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.1573 g/cm3 |
| Melting point | −51 °C (−60 °F; 222 K) |
| Boiling point | 175–176 °C (347–349 °F; 448–449 K) |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H227, H300, H315, H319 | |
| P210, P264, P270, P280, P301+P310+P330, P302+P352, P305+P351+P338, P332+P313, P337+P313, P370+P378, P403+P235, P405, P501 | |
| Related compounds | |
Related compounds
|
4-Chlorobutyronitrile Propionitrile |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
3-Chloropropionitrile is an organic compound with the formula ClCH2CH2CN. A colorless liquid, it is prepared by the reaction of hydrogen chloride with acrylonitrile. It is used commercially as a precursor to the drug famotidine.[1]
It is an alkylating agent, as illustrated by its reaction with imidazoles to give the cyanoethylated imidazolium salts.[2] Similarly, it alkylates thiourea, en route to 3-mercaptopropionitrile.[3]
References
[edit]- ^ Pollak, Peter; Romeder, Gérard; Hagedorn, Ferdinand; Gelbke, Heinz-Peter (2000). "Nitriles". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_363. ISBN 3527306730.
- ^ Zhao, Dongbin; Fei, Zhaofu; Scopelliti, Rosario; Dyson, Paul J. (2004). "Synthesis and Characterization of Ionic Liquids Incorporating the Nitrile Functionality". Inorganic Chemistry. 43 (6): 2197–2205. doi:10.1021/ic034801p. PMID 15018545.
- ^ R. Eric Gerber; Carlos Hasbun; Larisa G. Dubenko; Mei Fong King & Donald E. Bierer (2000). "β-Mercaptopropionitrile". Organic Syntheses. 77: 186. doi:10.15227/orgsyn.077.0186.
