Chlorophyll: Difference between revisions
m Reverted edits by 39.55.115.201 (talk) to last version by Materialscientist |
Tag: blanking |
||
| Line 32: | Line 32: | ||
</gallery> |
</gallery> |
||
Green Shieeit |
|||
==Chlorophyll and photosynthesis== |
|||
Chlorophyll is vital for [[photosynthesis]], which allows plants to absorb energy from light.<ref>{{cite web | url=http://biology.clc.uc.edu/courses/bio104/photosyn.htm | title =Photosynthesis | author =Carter, J. Stein |publisher=[[University of Cincinnati]]| year =1996}}</ref> |
|||
Chlorophyll molecules are specifically arranged in and around [[photosystem]]s that are embedded in the [[thylakoid]] membranes of [[chloroplast]]s.<ref>{{cite book | url=http://www.nature.com/scitable/topicpage/photosynthetic-cells-14025371 | title =Essentials of Cell Biology|chapter=Unit 1.3. Photosynthetic Cells | author =Nature | publisher =nature.com | date =July 5, 2013 }}</ref> In these complexes, chlorophyll serves two primary functions. The function of the vast majority of chlorophyll (up to several hundred molecules per photosystem) is to absorb light and transfer that light energy by [[resonance energy transfer]] to a specific chlorophyll pair in the [[reaction center]] of the photosystems. |
|||
The two currently accepted photosystem units are [[Photosystem II]] and [[Photosystem I]], which have their own distinct reaction center chlorophylls, named P680 and P700, respectively. These pigments are named after the wavelength (in [[nanometer]]s) of their red-peak absorption maximum. The identity, function and spectral properties of the types of chlorophyll in each photosystem are distinct and determined by each other and the protein structure surrounding them. Once extracted from the protein into a solvent (such as [[acetone]] or [[methanol]]),<ref name=Marker1972> |
|||
{{Cite journal |
|||
| last = Marker |
|||
| first = A. F. H. |
|||
| year = 1972 |
|||
| title = The use of acetone and methanol in the estimation of chlorophyll in the presence of phaeophytin |
|||
| journal = Freshwater Biology |
|||
| volume = 2 |
|||
| page = 361 |
|||
| doi = 10.1111/j.1365-2427.1972.tb00377.x |
|||
| issue = 4 |
|||
}}</ref><ref name=Jeffrey1969> |
|||
{{Cite journal |
|||
| last = Jeffrey |
|||
| first = S. W. |
|||
| author2 = Shibata, Kazuo |
|||
|date=February 1969 |
|||
| title = Some Spectral Characteristics of Chlorophyll c from Tridacna crocea Zooxanthellae |
|||
| journal = [[Biological Bulletin]] |
|||
| publisher = [[Marine Biological Laboratory]] |
|||
| volume = 136 |
|||
| issue = 1 |
|||
| pages = 54–62 |
|||
| doi = 10.2307/1539668| jstor = 1539668 |
|||
}}</ref><ref> |
|||
{{cite web |
|||
| author = Gilpin, Linda |
|||
| date = 21 March 2001 |
|||
| title = Methods for analysis of benthic photosynthetic pigment |
|||
| publisher = School of Life Sciences, [[Napier University]] |
|||
| url = http://web.archive.org/web/20080414215124/http://www.lifesciences.napier.ac.uk/teaching/MB/benchl01.html |
|||
| accessdate = 2010-07-17 |
|||
}}</ref> these chlorophyll pigments can be separated in a simple [[paper chromatography]] experiment and, based on the number of polar groups between [[chlorophyll a]] and [[chlorophyll b]], will chemically separate out on the paper. |
|||
The function of the reaction center chlorophyll is to use the energy absorbed by and transferred to it from the other chlorophyll pigments in the photosystems to undergo a charge separation, a specific [[redox]] reaction in which the chlorophyll donates an [[electron]] into a series of molecular intermediates called an [[electron transport chain]]. The charged reaction center chlorophyll (P680<sup>+</sup>) is then reduced back to its ground state by accepting an electron. In Photosystem II, the electron that reduces P680<sup>+</sup> ultimately comes from the oxidation of water into O<sub>2</sub> and H<sup>+</sup> through several intermediates. This reaction is how photosynthetic organisms such as plants produce O<sub>2</sub> gas, and is the source for practically all the O<sub>2</sub> in Earth's atmosphere. Photosystem I typically works in series with Photosystem II; thus the P700<sup>+</sup> of Photosystem I is usually reduced, via many intermediates in the thylakoid membrane, by electrons ultimately from Photosystem II. Electron transfer reactions in the thylakoid membranes are complex, however, and the source of electrons used to reduce P700<sup>+</sup> can vary. |
|||
The electron flow produced by the reaction center chlorophyll pigments is used to shuttle H<sup>+</sup> ions across the thylakoid membrane, setting up a [[chemiosmosis|chemiosmotic]] potential used mainly to produce [[Adenosine triphosphate|ATP]] chemical energy; and those electrons ultimately reduce NADP<sup>+</sup> to [[NADPH]], a universal [[redox|reductant]] used to reduce CO<sub>2</sub> into sugars as well as for other biosynthetic reductions. |
|||
Reaction center chlorophyll–protein complexes are capable of directly absorbing light and performing charge separation events without other chlorophyll pigments, but the absorption cross section (the likelihood of absorbing a photon under a given light intensity) is small. Thus, the remaining chlorophylls in the photosystem and antenna pigment protein complexes associated with the photosystems all cooperatively absorb and funnel light energy to the reaction center. Besides chlorophyll ''a'', there are other pigments, called [[accessory pigment]]s, which occur in these pigment–protein antenna complexes. |
|||
A green sea slug, ''[[Elysia chlorotica]]'', has been found to use the chlorophyll it has eaten to perform photosynthesis for itself. This process is known as [[kleptoplasty]], and no other animal has been found to have this ability. |
|||
===Why green and not black?=== |
|||
[[File:Black Pearl Plant.jpg|thumb|right|Black plants (such as this [[Black pearl pepper]]) can absorb more radiation, and yet most plants are green]] |
|||
It still is unclear exactly why plants have mostly evolved to be green. Green plants reflect mostly green and near-green light to viewers rather than absorbing it. Other parts of the system of [[photosynthesis]] still allow green plants to use the green light spectrum (e.g. through a light-trapping [[leaf]] structure, [[carotenoids]], etc.). Green plants do not use a large part of the visible spectrum as efficiently as possible. A black plant can absorb more radiation, and this could be very useful, if extra heat produced is effectively disposed of (e.g. some plants must close their openings, called [[stoma]]ta, on hot days to avoid losing too much water, which leaves only conduction, convection, and radiative heat-loss as solutions).<ref>{{cite book|last=Taiz, Zeiger|title=Plant Physiology|year=2002|publisher=Sinauer Associates|isbn=0-87893-823-0|page=181}}</ref> The question becomes why the only light-absorbing molecule used for power in plants is green and not simply black. |
|||
The biologist John Berman has offered the opinion that evolution is [[argument from poor design|not an engineering process]], and so it is often subject to various limitations that an [[engineer]] or other designer is not. Even '''''if''''' black leaves were better, evolution's limitations can prevent species from climbing to the absolute highest peak on the [[fitness landscape]]. Berman wrote that achieving pigments that work better than chlorophyll could be very difficult. In fact, all higher plants ([[embryophyte]]s) are thought to have evolved from a common ancestor that is a sort of [[green algae|green alga]] – with the idea being that chlorophyll has evolved only once.<ref name=JohnBerman>[http://www.askabiologist.org.uk/answers/viewtopic.php?id=3837 askabiologist.org/uk, Jonathan Max Berman, "Why did plants evolve green, not black?"]. Askabiologist.org.uk. Retrieved on 2012-04-15.</ref> |
|||
Shil DasSarma, a microbial geneticist at the [[University of Maryland]], has pointed out that species of [[archaea]] do use another light-absorbing molecule, [[retinal]], to extract power from the green spectrum. He described the view of some scientists that such green-light-absorbing archae once dominated the earth environment. This could have left open a "niche" for green organisms that would absorb the other wavelengths of sunlight. This is just a possibility, and Berman wrote that scientists are still not convinced of any one explanation.<ref>[http://www.livescience.com/1398-early-earth-purple-study-suggests.html Early Earth Was Purple, Study Suggests]. Livescience.com (2007-04-10). Retrieved on 2012-04-15.</ref> |
|||
Astronomer and mathematician [[Fred Hoyle]] conjectured that chlorophyll was likely to be an interstellar molecule, pointing out the similarities of its light absorbing properties to interstellar dust.<ref>{{cite book|last=Hoyle, Fred|title=Intelligent Universe|year=1988|publisher=Book Sales|isbn=978-0030700835|page=126}}</ref> |
|||
==Chemical structure== |
==Chemical structure== |
||
Revision as of 15:13, 19 March 2014
Chlorophyll (also chlorophyl) is a green pigment found in cyanobacteria and the chloroplasts of algae and plants.[2] Its name is derived from the Greek words χλωρός, chloros ("green") and φύλλον, phyllon ("leaf").[3] Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to absorb energy from light. Chlorophyll absorbs light most strongly in the blue portion of the electromagnetic spectrum, followed by the red portion. Conversely, it is a poor absorber of green and near-green portions of the spectrum, hence the green color of chlorophyll-containing tissues.[4] Chlorophyll was first isolated by Joseph Bienaimé Caventou and Pierre Joseph Pelletier in 1817.[5]
Gallery
-
Chlorophyll gives leaves their green color and absorbs light that is used in photosynthesis.
-
Chlorophyll is found in high concentrations in chloroplasts of plant cells.
-
Absorption maxima of chlorophylls against the spectrum of white light.[citation needed]
-
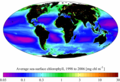
SeaWiFS-derived average sea surface chlorophyll for the period 1998 to 2006.
Green Shieeit
Chemical structure
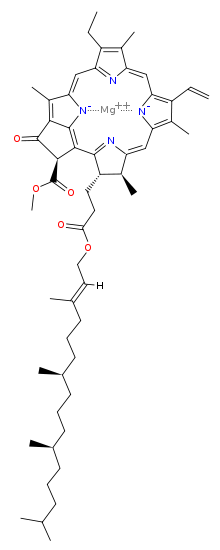
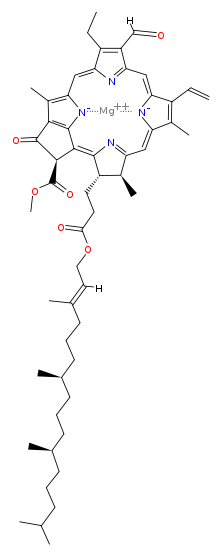
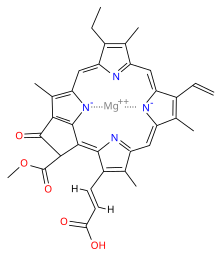
Chlorophyll is a chlorin pigment, which is structurally similar to and produced through the same metabolic pathway as other porphyrin pigments such as heme. At the center of the chlorin ring is a magnesium ion. At the time of its discovery in the early 1900s, this was the first time that this element had been detected in living tissue.[6] For the structures depicted in this article, some of the ligands attached to the Mg2+ center are omitted for clarity. The chlorin ring can have several different side chains, usually including a long phytol chain. There are a few different forms that occur naturally, but the most widely distributed form in terrestrial plants is chlorophyll a. After initial work done by German chemist Richard Willstätter spanning from 1905 to 1915, the general structure of chlorophyll a was elucidated by Hans Fischer in 1940. By 1960, when most of the stereochemistry of chlorophyll a was known, Robert Burns Woodward published a total synthesis of the molecule.[6][7] In 1967, the last remaining stereochemical elucidation was completed by Ian Fleming,[8] and in 1990 Woodward and co-authors published an updated synthesis.[9] Chlorophyll f was announced to be present in cyanobacteria and other oxygenic microorganisms that form stromatolites in 2010;[10][11] a molecular formula of C55H70O6N4Mg and a structure of (2-formyl)-chlorophyll a were deduced based on NMR, optical and mass spectra.[12] The different structures of chlorophyll are summarized below:[citation needed]
| Chlorophyll a | Chlorophyll b | Chlorophyll c1 | Chlorophyll c2 | Chlorophyll d | Chlorophyll f | |
|---|---|---|---|---|---|---|
| Molecular formula | C55H72O5N4Mg | C55H70O6N4Mg | C35H30O5N4Mg | C35H28O5N4Mg | C54H70O6N4Mg | C55H70O6N4Mg |
| C2 group | -CH3 | -CH3 | -CH3 | -CH3 | -CH3 | -CHO |
| C3 group | -CH=CH2 | -CH=CH2 | -CH=CH2 | -CH=CH2 | -CHO | -CH=CH2 |
| C7 group | -CH3 | -CHO | -CH3 | -CH3 | -CH3 | -CH3 |
| C8 group | -CH2CH3 | -CH2CH3 | -CH2CH3 | -CH=CH2 | -CH2CH3 | -CH2CH3 |
| C17 group | -CH2CH2COO-Phytyl | -CH2CH2COO-Phytyl | -CH=CHCOOH | -CH=CHCOOH | -CH2CH2COO-Phytyl | -CH2CH2COO-Phytyl |
| C17-C18 bond | Single (chlorin) |
Single (chlorin) |
Double (porphyrin) |
Double (porphyrin) |
Single (chlorin) |
Single (chlorin) |
| Occurrence | Universal | Mostly plants | Various algae | Various algae | Cyanobacteria | Cyanobacteria |
 |
 |
 |
 |
 |
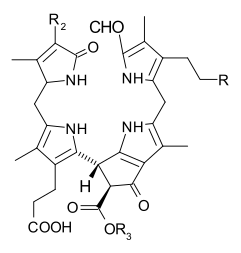
When leaves degreen in the process of plant senescence, chlorophyll is converted to a group of colourless tetrapyrroles known as nonfluorescent chlorophyll catabolites (NCC's) with the general structure:
These compounds have also been identified in several ripening fruits.[13]
Spectrophotometry

Measurement of the absorption of light is complicated by the solvent used to extract it from plant material, which affects the values obtained,
- In diethyl ether, chlorophyll a has approximate absorbance maxima of 430 nm and 662 nm, while chlorophyll b has approximate maxima of 453 nm and 642 nm.[14]
- The absorption peaks of chlorophyll a are at 665 nm and 465 nm. Chlorophyll a fluoresces at 673 nm (maximum) and 726 nm. The peak molar absorption coefficient of chlorophyll a exceeds 105 M−1 cm−1, which is among the highest for small-molecule organic compounds.[citation needed]
- In 90% acetone-water, the peak absorption wavelengths of chlorophyll a are 430 nm and 664 nm; peaks for chlorophyll b are 460 nm and 647 nm; peaks for chlorophyll c1 are 442 nm and 630 nm; peaks for chlorophyll c2 are 444 nm and 630 nm; peaks for chlorophyll d are 401 nm, 455 nm and 696 nm.[15]

By measuring the absorption of light in the red and far red regions it is possible to estimate the concentration of chlorophyll within a leaf.[16]
While absorption instruments have been proven over time to work well as a non-destructive test on many types of samples, they do have limitations. Absorption technique limitations:
- The sample to be measured must completely cover the instrument’s aperture, with no holes.
- Samples must be thin enough to allow transmission of the measuring wavelengths of light.
- Surfaces must be relatively flat and uniform.
- Variable fluorescence (the Kautsky induction effect) caused by the red wavelength of absorption instruments, limits the repeatability of measurements at the same location.
- Selection of the measuring area, on smaller leaves, can cause measurement variations due to internal leaf structure variation. Veins and midribs can cause significant variation on samples.
- Wavelengths used in absorption methods, typically limit linear correlation with chemical chlorophyll content methods to concentration levels below 300 mg/m2.[17]
As a result, absorption instruments do not work with conifer needles, turf grasses, Arabidopsis leaves, moss, most CAM plants such as prickly pear cactus, and Agave, fruit, stems, petioles, lichens, and algae on rocks. Furthermore, it is difficult to get reliable readings on very small leaf plants such as immature rice and wheat.
A superior method is to measure the chlorophyll content using chlorophyll fluorescence. Gitelson (1999) states, "The ratio between chlorophyll fluorescence, at 735 nm and the wavelength range 700nm to 710 nm, F735/F700 was found to be linearly proportional to the chlorophyll content (with determination coefficient, r2, more than 0.95) and thus this ratio can be used as a precise indicator of chlorophyll content in plant leaves."[17] Non-destructive, hand-held meters use this effect to measure the chlorophyll content of samples where traditional absorbance techniques cannot be used. This new method has many advantages over older, absorption techniques. These include:-

- Samples do not need to fill the measuring aperture, so small samples can be measured.
- Samples do not need to allow transmission of the measuring beam, so thick samples can be measured.
- Surfaces do not need to be flat and uniform, so difficult plant morphologies can be measured.
- No Kautsky induction effect, so measurements can be repeated rapidly at the same site.
- Small sample area, so naturally occurring structures like mid ribs and veins can be avoided.
- Higher measuring range (675 mg/m2, as opposed to 300 mg/m2 with absorption techniques), so many more samples can be measured.
By measuring chlorophyll fluorescence, plant ecophysiology can be investigated. Chlorophyll fluorometers are used by plant researchers to assess plant stress.
Biosynthesis
In plants, chlorophyll may be synthesized from succinyl-CoA and glycine, although the immediate precursor to chlorophyll a and b is protochlorophyllide. In Angiosperm plants, the last step, conversion of protochlorophyllide to chlorophyll, is light-dependent and such plants are pale (etiolated) if grown in the darkness. Non-vascular plants and green algae have an additional light-independent enzyme and grow green in the darkness instead.
Chlorophyll itself is bound to proteins and can transfer the absorbed energy in the required direction. Protochlorophyllide occurs mostly in the free form and, under light conditions, acts as a photosensitizer, forming highly toxic free radicals. Hence, plants need an efficient mechanism of regulating the amount of chlorophyll precursor. In angiosperms, this is done at the step of aminolevulinic acid (ALA), one of the intermediate compounds in the biosynthesis pathway. Plants that are fed by ALA accumulate high and toxic levels of protochlorophyllide; so do the mutants with the damaged regulatory system.[18]
Chlorosis is a condition in which leaves produce insufficient chlorophyll, turning them yellow. Chlorosis can be caused by a nutrient deficiency of iron—called iron chlorosis—or by a shortage of magnesium or nitrogen. Soil pH sometimes plays a role in nutrient-caused chlorosis; many plants are adapted to grow in soils with specific pH levels and their ability to absorb nutrients from the soil can be dependent on this.[19] Chlorosis can also be caused by pathogens including viruses, bacteria and fungal infections, or sap-sucking insects.
Complementary light absorbance of anthocyanins with chlorophylls

Anthocyanins are other plant pigments. The absorbance pattern responsible for the red color of anthocyanins may be complementary to that of green chlorophyll in photosynthetically active tissues such as young Quercus coccifera leaves. It may protect the leaves from attacks by plant eaters that may be attracted by green color.[20]
Culinary use
Chlorophyll is registered as a food additive (colorant), and its E number is E140. Chefs use chlorophyll to color a variety of foods and beverages green, such as pasta and absinthe.[21] Chlorophyll is not soluble in water, and it is first mixed with a small quantity of vegetable oil to obtain the desired solution. Extracted liquid chlorophyll was considered to be unstable and always denatured until 1997,[citation needed] when Frank S. & Lisa Sagliano used freeze-drying of liquid chlorophyll at the University of Florida and stabilized it as a powder, preserving it for future use.[22]
Alternative medicine
There are many claims that are made about the healing properties of chlorophyll but most have been disproved or are exaggerated by the companies that are marketing them. Quackwatch, a website dedicated to debunking false medical claims, has a quote from Teledo Blade (1952) which claims [23] "Chlorophyll Held Useless As Body Deodorant",[24] but later has John C. Kephart pointing out "No deodorant effect can possibly occur from the quantities of chlorophyll put in products such as gum, foot powder, cough drops, etc. To be effective, large doses must be given internally".[25]
See also
- Bacteriochlorophyll, related compounds in phototrophic bacteria
- Chlorophyll a, an essential chlorophyll pigment
- Chlorophyll b, also an essential chlorophyll pigment
- Chlorophyllin, a semi-synthetic derivative of chlorophyll
- Deep chlorophyll maximum
- Grow light, a lamp that promotes photosynthesis
References
- ^ Chlorophyll : Global Maps. Earthobservatory.nasa.gov. Retrieved on 2014-02-02.
- ^ May, Paul. "Chlorophyll". University of Bristol.
- ^ "chlorophyll". Online Etymology Dictionary.
- ^ Chlorophyll molecules are specifically arranged in and around photosystems that are embedded in the thylakoid membranes of chloroplasts. Two types of chlorophyll exist in the photosystems: chlorophyll a and b.
Speer, Brian R. (1997). "Photosynthetic Pigments". UCMP Glossary (online). University of California Museum of Paleontology. Retrieved 2010-07-17.
{{cite web}}: External link in|work= - ^ Delépine, Marcel [in French] (September 1951). "Joseph Pelletier and Joseph Caventou". Journal of Chemical Education. 28 (9): 454. Bibcode:1951JChEd..28..454D. doi:10.1021/ed028p454.
- ^ a b Motilva, Maria-José (2008). "Chlorophylls – from functionality in food to health relevance". 5th Pigments in Food congress- for quality and health. University of Helsinki. ISBN 978-952-10-4846-3.
{{cite book}}:|format=requires|url=(help) - ^ Woodward, R. B.; Ayer, W. A.; Beaton, J. M.; Bickelhaupt, F.; Bonnett, R.; Buchschacher, P.; Closs, G. L.; Dutler, H.; Hannah, J.; et al. (July 1960). "The total synthesis of chlorophyll". Journal of the American Chemical Society. 82 (14): 3800–3802. doi:10.1021/ja01499a093.
- ^ Fleming, Ian (14 October 1967). "Absolute Configuration and the Structure of Chlorophyll". Nature. 216 (5111): 151–152. Bibcode:1967Natur.216..151F. doi:10.1038/216151a0.
- ^ Woodward, R. B.; Ayer, William A.; Beaton, John M.; Bickelhaupt, Friedrich; Bonnett, Raymond; Buchschacher, Paul; Closs, Gerhard L.; Dutler, Hans; Hannah, John; et al. (1990). "The total synthesis of chlorophyll a" (PDF). Tetrahedron. 46 (22): 7599–7659. doi:10.1016/0040-4020(90)80003-Z.
- ^ Jabr, Ferris (August 19, 2010) A New Form of Chlorophyll?. Scientific American. Retrieved on 2012-04-15.
- ^ Infrared chlorophyll could boost solar cells. New Scientist. August 19, 2010. Retrieved on 2012-04-15.
- ^ Chen, Min; Schliep, Martin; Willows, Robert D.; Cai, Zheng-Li; Neilan, Brett A.; Scheer, Hugo (September 2010). "A Red-Shifted Chlorophyll". Science. 329 (5997): 1318–1319. Bibcode:2010Sci...329.1318C. doi:10.1126/science.1191127. PMID 20724585.
- ^ Müller, Thomas; Ulrich, Markus; Ongania, Karl-Hans; Kräutler, Bernhard (2007). "Colorless Tetrapyrrolic Chlorophyll Catabolites Found in Ripening Fruit Are Effective Antioxidants". Angewandte Chemie. 46 (45): 8699–8702. doi:10.1002/anie.200703587. PMC 2912502. PMID 17943948.
- ^ Gross, Jeana (1991). Pigments in vegetables: chlorophylls and carotenoids. Van Nostrand Reinhold, ISBN 0442006578.
- ^ Larkum, edited by Anthony W. D. Larkum, Susan E. Douglas & John A. Raven (2003). Photosynthesis in algae. London: Kluwer. ISBN 0-7923-6333-7.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Cate, Thomas; Perkins, T. D. (September 2003). "Joseph Pelletier and Joseph Caventou". Journal of Tree Physiology. 23 (15): 1077–1079. doi:10.1093/treephys/23.15.1077.
- ^ a b Gitelson, Anatoly A; Buschmann, Claus; Lichtenthaler, Hartmut K (1999). "The Chlorophyll Fluorescence Ratio F735/F700 as an Accurate Measure of the Chlorophyll Content in Plants". Remote Sensing of Environment. 69 (3): 296. doi:10.1016/S0034-4257(99)00023-1.
- ^
Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp R, Apel K. (23 October 2001). "FLU: A negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana". Proceedings of the National Academy of Sciences. 98 (22): 12826–12831. doi:10.1073/pnas.98.22.12826. PMC 60138. PMID 11606728.
{{cite journal}}: Unknown parameter|doi_inactivedate=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Duble, Richard L. "Iron Chlorosis in Turfgrass". Texas A&M University. Retrieved 2010-07-17.
- ^ Karageorgou, P.; Manetas, Y. (2006). "The importance of being red when young: Anthocyanins and the protection of young leaves of Quercus coccifera from insect herbivory and excess light". Tree Physiology. 26 (5): 613–21. doi:10.1093/treephys/26.5.613. PMID 16452075.
- ^ Adams, Jad (2004). Hideous absinthe : a history of the devil in a bottle. Madison, Wisconsin: University of Wisconsin Press. p. 22. ISBN 978-0-299-20000-8.
- ^ US patent 5820916, Sagliano, Frank S. & Sagliano, Elizabeth A., "Method for growing and preserving wheat grass nutrients and products thereof", issued 1998-10-13
- ^ Amazing Claims for Chlorophyll, Quackwatch
- ^ Ubell, Earl (December 10, 1952) "Chlorophyll Held Useless As Body Deodorant", Toledo Blade
- ^ Kephart, John C. (1955). "Chlorophyll derivatives—Their chemistry? Commercial preparation and uses". Journal of Ecological Botany. 9: 3. doi:10.1007/BF02984956.