Chlorophyll: Difference between revisions
m Reverted edits by Tylergibson3440 (talk) to last version by Logan |
|||
| Line 10: | Line 10: | ||
==Chlorophyll and photosynthesis== |
==Chlorophyll and photosynthesis== |
||
You can read all of this or say it simply it's the stuff that makes plants green. |
|||
Chlorophyll is vital for [[photosynthesis]], which allows plants to obtain energy from light. |
Chlorophyll is vital for [[photosynthesis]], which allows plants to obtain energy from light. |
||
Revision as of 02:55, 7 June 2009



Template:FixHTML Chlorophyll is a green pigment found in most plants, algae, and cyanobacteria. Its name is derived from the Greek χλωρός (chloros "green") and φύλλον (phyllon "leaf"). Chlorophyll absorbs light most strongly in the blue and red but poorly in the green portions of the electromagnetic spectrum, hence the green colour of chlorophyll-containing tissues such as plant leaves.[1]
Chlorophyll and photosynthesis
You can read all of this or say it simply it's the stuff that makes plants green.
Chlorophyll is vital for photosynthesis, which allows plants to obtain energy from light.
Chlorophyll molecules are specifically arranged in and around pigment protein complexes called photosystems which are embedded in the thylakoid membranes of chloroplasts. In these complexes, chlorophyll serves two primary functions. The function of the vast majority of chlorophyll (up to several hundred molecules per photosystem) is to absorb light and transfer that light energy by resonance energy transfer to a specific chlorophyll pair in the reaction center of the photosystems. Because of chlorophyll’s selectivity regarding the wavelength of light it absorbs, areas of a leaf containing the molecule will appear green.
The two currently accepted photosystem units are Photosystem II and Photosystem I, which have their own distinct reaction center chlorophylls, named P680 and P700, respectively.[2] These pigments are named after the wavelength (in nanometers) of their red-peak absorption maximum. The identity, function and spectral properties of the types of chlorophyll in each photosystem are distinct and determined by each other and the protein structure surrounding them. Once extracted from the protein into a solvent (such as acetone or methanol),[3][4][5] these chlorophyll pigments can be separated in a simple paper chromatography experiment, and, based on the number of polar groups between chlorophyll a and chlorophyll b, will chemically separate out on the paper.
The function of the reaction center chlorophyll is to use the energy absorbed by and transferred to it from the other chlorophyll pigments in the photosystems to undergo a charge separation, a specific redox reaction in which the chlorophyll donates an electron into a series of molecular intermediates called an electron transport chain. The charged reaction center chlorophyll (P680+) is then reduced back to its ground state by accepting an electron. In Photosystem II, the electron which reduces P680+ ultimately comes from the oxidation of water into O2 and H+ through several intermediates. This reaction is how photosynthetic organisms like plants produce O2 gas, and is the source for practically all the O2 in Earth's atmosphere. Photosystem I typically works in series with Photosystem II, thus the P700+ of Photosystem I is usually reduced, via many intermediates in the thylakoid membrane, by electrons ultimately from Photosystem II. Electron transfer reactions in the thylakoid membranes are complex, however, and the source of electrons used to reduce P700+ can vary.
The electron flow produced by the reaction center chlorophyll pigments is used to shuttle H+ ions across the thylakoid membrane, setting up a chemiosmotic potential mainly used to produce ATP chemical energy, and those electrons ultimately reduce NADP+ to NADPH a universal reductant used to reduce CO2 into sugars as well as for other biosynthetic reductions.
Reaction center chlorophyll-protein complexes are capable of directly absorbing light and performing charge separation events without other chlorophyll pigments, but the absorption cross section (the likelihood of absorbing a photon under a given light intensity) is small. Thus, the remaining chlorophylls in the photosystem and antenna pigment protein complexes associated with the photosystems all cooperatively absorb and funnel light energy to the reaction center. Besides chlorophyll a, there are other pigments, called accessory pigments, which occur in these pigment-protein antenna complexes.
Chemical structure
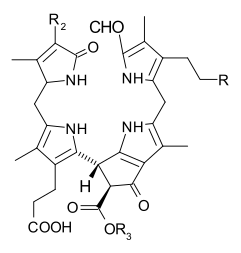
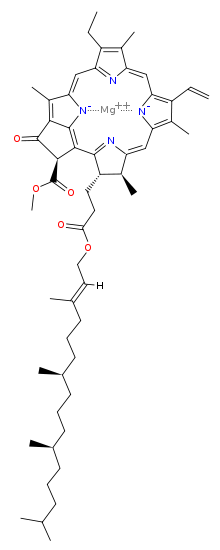
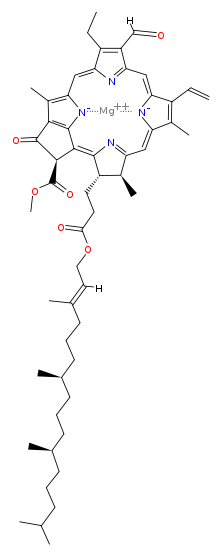
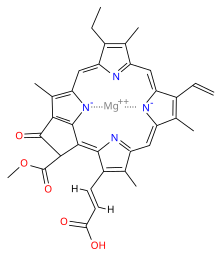
Chlorophyll is a chlorin pigment, which is structurally similar to and produced through the same metabolic pathway as other porphyrin pigments such as heme. At the center of the chlorin ring is a magnesium ion. The chlorin ring can have several different side chains, usually including a long phytol chain. There are a few different forms that occur naturally, but the most widely distributed form in terrestrial plants is chlorophyll a. The general structure of chlorophyll a was elucidated by Hans Fischer in 1940, and by 1960, when most of the stereochemistry of chlorophyll a was known, Robert Burns Woodward published a total synthesis of the molecule as then known.[6] In 1967, the last remaining stereochemical elucidation was completed by Ian Fleming,[7] and in 1990 Woodward and co-authors published an updated synthesis.[8]
The different structures of chlorophyll are summarized below:
| Chlorophyll a | Chlorophyll b | Chlorophyll c1 | Chlorophyll c2 | Chlorophyll d | |
|---|---|---|---|---|---|
| Molecular formula | C55H72O5N4Mg | C55H70O6N4Mg | C35H30O5N4Mg | C35H28O5N4Mg | C54H70O6N4Mg |
| C3 group | -CH=CH2 | -CH=CH2 | -CH=CH2 | -CH=CH2 | -CHO |
| C7 group | -CH3 | -CHO | -CH3 | -CH3 | -CH3 |
| C8 group | -CH2CH3 | -CH2CH3 | -CH2CH3 | -CH=CH2 | -CH2CH3 |
| C17 group | -CH2CH2COO-Phytyl | -CH2CH2COO-Phytyl | -CH=CHCOOH | -CH=CHCOOH | -CH2CH2COO-Phytyl |
| C17-C18 bond | Single | Single | Double | Double | Single |
| Occurrence | Universal | Mostly plants | Various algae | Various algae | Cyanobacteria |
 |
 |
 |
 |
 |
When leaves degreen in the process of plant senescence chlorophyll is converted to a group of colourless tetrapyrroles known as nonfluorescent chlorophyll catabolites (NCC's) with the general structure:
These compounds have also been identified in several ripening fruits.[9]
Spectrophotometry

Measurement of the absorption of light is complicated by the solvent used to extract it from plant material, which affects the values obtained,
- In diethyl ether, chlorophyll a has approximate absorbance maxima of 430 nm and 662 nm, while chlorophyll b has approximate maxima of 453 nm and 642 nm.[10]
- The absorption peaks of chlorophyll a are at 665 nm and 465 nm. Chlorophyll a fluoresces at 673 nm (maximum) and 726 nm. The peak molar absorption coefficient of chlorophyll a exceeds 105 M−1 cm−1, which is among the highest for organic compounds. [source needed]
Biosynthesis
In plants, chlorophyll may be synthesized from succinyl-CoA and glycine, although the immediate precursor to chlorophyll a and b is protochlorophyllide. In Angiosperms, the last step, conversion of protochlorophyllide to chlorophyll, is light-dependent and such plants are pale (etiolated) if grown in the darkness. Non-vascular plants and green algae have an additional light-independent enzyme and grow green in the darkness as well.
Chlorophyll itself is bound to proteins and can transfer the absorbed energy in the required direction. Protochlorophyllide, differently, mostly occur in the free form and under light conditions act as photosensitizer, forming highly toxic free radicals. Hence plants need an efficient mechanism of regulating the amount of chlorophyll precursor. In angiosperms, this is done at the step of aminolevulinic acid (ALA), one of the intermediate compounds in the biosynthesis pathway. Plants that are fed by ALA accumulate high and toxic levels of protochlorophyllide, so do the mutants with the damaged regulatory system.[11]
Chlorosis is a condition in which leaves produce insufficient chlorophyll, turning them yellow. Chlorosis can be caused by a nutrient deficiency including iron - called iron chlorosis, or in a shortage of magnesium or nitrogen. Soil pH sometimes play a role in nutrient-caused chlorosis, many plants are adapted to grow in soils with specific pHs and their ability to absorb nutrients from the soil can be dependent on the soil pH.[12] Chlorosis can also be caused by pathogens including viruses, bacteria and fungal infections or sap sucking insects.
Culinary use
Chefs use chlorophyll to colour a variety of foods and beverages green, such as pasta and absinthe.[13] Chlorophyll is not soluble in water and is first mixed with a small quantity of oil to obtain the desired result.
See also
- Bacteriochlorophyll, related compounds in phototrophic bacteria
- Chlorophyllin, a semi-synthetic derivative of chlorophyll
- Copper chlorophyll
- Grow light, a lamp that promotes photosynthesis
References
- ^ Speer, Brian R. (1997). "Photosynthetic Pigments" in UCMP Glossary (online). University of California, Berkeley Museum of Paleontology. Verified availability March 12, 2007.
- ^ Green, 1984
- ^ Marker, A. F. H. (1972), "The use of acetone and methanol in the estimation of chlorophyll in the presence of phaeophytin", Freshwater Biology, 2: 361, doi:10.1111/j.1365-2427.1972.tb00377.x
- ^ Jeffrey, S. W. (1969), "SOME SPECTRAL CHARACTERISTICS OF CHLOROPHYLL c FROM TRIDACNA CROCEA ZOOXANTHELLAE", Biol Bull, 136: 54–62, doi:10.2307/1539668
{{citation}}: Unknown parameter|month=ignored (help) - ^ http://www.lifesciences.napier.ac.uk/teaching/MB/benchl01.html
- ^ R. B. Woodward, W. A. Ayer, J. M. Beaton, F. Bickelhaupt, R. Bonnett, P. Buchschacher, G. L. Closs, H. Dutler, J. Hannah, F. P. Hauck, S. Itô, A. Langemann, E. Le Goff, W. Leimgruber, W. Lwowski, J. Sauer, Z. Valenta, and H. Volz (1960). "The total synthesis of chlorophyll" (PDF). Journal of the American Chemical Society. 82: 3800–3802. doi:10.1021/ja01499a093.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ian Fleming (1967). "Absolute Configuration and the Structure of Chlorophyll". Nature. 216: 151–152. doi:10.1038/216151a0.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Robert Burns Woodward, William A. Ayer, John M. Beaton, Friedrich Bickelhaupt, Raymond Bonnett, Paul Buchschacher, Gerhard L. Closs, Hans Dutler, John Hannah, Fred P. Hauck; et al. (1990). "The total synthesis of chlorophyll a". Tetrahedron. 46 (22): 7599–7659. doi:10.1016/0040-4020(90)80003-Z.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Colourless Tetrapyrrolic Chlorophyll Catabolites Found in Ripening Fruit Are Effective Antioxidants Thomas Muller, Markus Ulrich, Karl-Hans Ongania, and Bernhard Krautler Angew. Chem. Int. Ed. 2007, 46, 8699 –8702 doi:10.1002/anie.200703587
- ^ Gross, 1991
- ^ http://www.pnas.org/content/98/22/12826.abstract?ck=nck
- ^ Iron Chlorosis in Turfgrass
- ^ Adams, Jad (2004), Hideous absinthe : a history of the devil in a bottle, Madison, Wisconsin: University of Wisconsin Press, p. 22, ISBN 9780299200008