Chlorogenic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
(1S,3R,4R,5R)-3-{[(2E)-3-(3,4-Dihydroxyphenyl)prop-2-enoyl]oxy}-1,4,5-trihydroxycyclohexane-1-carboxylic acid | |
| Other names
3-(3,4-Dihydroxycinnamoyl)quinate
3-(3,4-Dihydroxycinnamoyl)quinic acid 3-Caffeoylquinate 3-Caffeoylquinic acid 3-CQA 3-O-Caffeoylquinic acid Chlorogenate Chlorogenic acid Heriguard 3-trans-Caffeoylquinic acid 5-O-Caffeoylquinic acid | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H18O9 | |
| Molar mass | 354.311 g·mol−1 |
| Density | 1.28 g/cm3 |
| Melting point | 207 to 209 °C (405 to 408 °F; 480 to 482 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
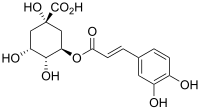
Chlorogenic acid (CGA) is the ester of caffeic acid and (−)-quinic acid, functioning as an intermediate in lignin biosynthesis.[1] The term chlorogenic acids refers to a related polyphenol family of esters, including hydroxycinnamic acids (caffeic acid, ferulic acid and p-coumaric acid) with quinic acid.[2]
Despite the "chloro" of the name, chlorogenic acids contain no chlorine. Instead, the name comes from the Greek χλωρός (khloros, light green) and -γένος (genos, a suffix meaning "giving rise to"), pertaining to the green color produced when chlorogenic acids are oxidized.
Structural properties
[edit]Structurally, chlorogenic acid is the ester formed between caffeic acid and the 3-hydroxyl of L-quinic acid.[3][4] Isomers of chlorogenic acid include the caffeoyl ester at other hydroxyl sites on the quinic acid ring: 4-O-caffeoylquinic acid (cryptochlorogenic acid or 4-CQA) and 5-O-caffeoylquinic acid (neochlorogenic acid or 5-CQA). The epimer at position 1 has not yet been reported.[2]
Structures having more than one caffeic acid group are called isochlorogenic acids, and can be found in coffee.[5] There are several isomers, such as 3,4-dicaffeoylquinic acid and 3,5-dicaffeoylquinic acid.[6] and cynarine (1,5-dicaffeoylquinic acid)
Biosynthesis and natural occurrence
[edit]
The biosynthetic precursor to chlorogenic acid is 4-coumaroyl-CoA, containing a single hydroxyl group on the aryl ring, which in turn is produced from cinnamic acid. The hydroxylation of the coumaryl ester, i.e. installing the second hydroxy group, is catalyzed by a cytochrome P450 enzyme.[7]
Chlorogenic acid can be found in the bamboo Phyllostachys edulis,[8] as well as in many other plants,[9] such as the shoots of common heather (Calluna vulgaris).[10]
In food
[edit]Chlorogenic acid and the related compounds cryptochlorogenic acid, and neochlorogenic acid have been found in the leaves of Hibiscus sabdariffa.[11] Isomers of chlorogenic acid are found in potatoes.[12] Chlorogenic acid is present in the flesh of eggplants,[13] peaches,[14] prunes[15] and coffee beans.[16]
Research and safety
[edit]Chlorogenic acid is under preliminary research for its possible biological effects.[17][18][19]
Chlorogenic acid has not been approved as a prescription drug or food additive recognized as a safe ingredient for foods or beverages.[20] There is not enough evidence to determine whether it is safe or effective for human health, and its use in high doses, such as excessive consumption of green coffee, may have adverse effects.[21]
Nomenclature
[edit]The atom-numbering of chlorogenic acid can be ambiguous.[22] The order of numbering of atoms on the quinic acid ring was reversed in 1976 following IUPAC guidelines, with the consequence that 3-CQA became 5-CQA, and 5-CQA became 3-CQA. This article uses the original numbering, which was exclusive prior to 1976, (chlorogenic acid being 3-CQA, while neochlorogenic acid is 5-CQA). Thereafter researchers and manufacturers have been divided, with both numbering systems in use. Even the 1976 IUPAC recommendations are not entirely satisfactory when applied to some of the less common chlorogenic acids.[23]
References
[edit]- ^ Boerjan, Wout; Ralph, John; Baucher, Marie (2003). "Lignin biosynthesis". Annual Review of Plant Biology. 54: 519–546. doi:10.1146/annurev.arplant.54.031902.134938. PMID 14503002.
- ^ a b Clifford, M. N.; Johnston, K. L.; Knight, S.; Kuhnert, N. (2003). "Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids". Journal of Agricultural and Food Chemistry. 51 (10): 2900–2911. doi:10.1021/jf026187q. PMID 12720369.
- ^ Clifford, M. N. (1999). "Chlorogenic acids and other cinnamates – nature, occurrence and dietary burden". Journal of the Science of Food and Agriculture. 79 (3): 362–372. doi:10.1002/(SICI)1097-0010(19990301)79:3<362::AID-JSFA256>3.0.CO;2-D.
- ^ Clifford, Michael N.; Jaganath, Indu B.; Ludwig, Iziar A.; Crozier, Alan (2017). "Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity". Natural Product Reports. 34 (12): 1391–1421. doi:10.1039/C7NP00030H. PMID 29160894.
- ^ Barnes, H. M.; Feldman, J. R.; White, W. V. (1950). "Isochlorogenic Acid. Isolation from Coffee and Structure Studies". J. Am. Chem. Soc. 72 (9): 4178–4182. doi:10.1021/ja01165a095.
- ^ Corse, J.; Lundin, R. E.; Waiss, A. C. (May 1965). "Identification of several components of isochlorogenic acid". Phytochemistry. 4 (3): 527–529. Bibcode:1965PChem...4..527C. doi:10.1016/S0031-9422(00)86209-3.
- ^ Vogt, T. (2010). "Phenylpropanoid Biosynthesis". Molecular Plant. 3 (1): 2–20. doi:10.1093/mp/ssp106. PMID 20035037.
- ^ Kweon, Mee-Hyang; Hwang, Han-Joon; Sung, Ha-Chin (2001). "Identification and Antioxidant Activity of Novel Chlorogenic Acid Derivatives from Bamboo (Phyllostachys edulis)". Journal of Agricultural and Food Chemistry. 49 (20): 4646–4652. doi:10.1021/jf010514x. PMID 11600002.
- ^ Clifford, M. N. (2003). "14. The analysis and characterization of chlorogenic acids and other cinnamates". In Santos-Buelga, C.; Williamson, G. (eds.). Methods in Polyphenol Analysis. Cambridge: Royal Society of Chemistry. pp. 314–337. ISBN 978-0-85404-580-8.
- ^ Jalal, Mahbubul A. F.; Read, David J.; Haslam, E. (1982). "Phenolic composition and its seasonal variation in Calluna vulgaris". Phytochemistry. 21 (6): 1397–1401. Bibcode:1982PChem..21.1397J. doi:10.1016/0031-9422(82)80150-7.
- ^ Zhen, Jing; Villani, Thomas S.; Guo, Yue; Qi, Yadong; Chin, Kit; Pan, Min-Hsiung; Ho, Chi-Tang; Simon, James E.; Wu, Qingli (2016). "Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves". Food Chemistry. 190: 673–680. doi:10.1016/j.foodchem.2015.06.006. PMID 26213025.
- ^ Friedman, Mendel (1997). "Chemistry, Biochemistry, and Dietary Role of Potato Polyphenols. A Review". Journal of Agricultural and Food Chemistry. 45 (5): 1523–1540. doi:10.1021/jf960900s.
- ^ Luthria, Devanand L.; Mukhopadhyay, Sudarsan (2006). "Influence of Sample Preparation on Assay of Phenolic Acids from Eggplant". J. Agric. Food Chem. 54 (1): 41–47. doi:10.1021/jf0522457. PMID 16390175.
- ^ Cheng, G. W.; Crisosto, C. H. (September 1995). "Browning Potential, Phenolic Composition, and Polyphenoloxidase Activity of Buffer Extracts of Peach and Nectarine Skin Tissue". Journal of the American Society for Horticultural Science. 120 (5): 835–838. doi:10.21273/JASHS.120.5.835.
- ^ Stacewicz-Sapuntzakis, M.; Bowen, P. E.; Hussain, E. A.; Damayanti-Wood, B. I.; Farnsworth, N. R. (2001). "Chemical composition and potential health effects of prunes: a functional food?". Critical Reviews in Food Science and Nutrition. 41 (4): 251–286. doi:10.1080/20014091091814. PMID 11401245. S2CID 31159565.
- ^ Macheiner, Lukas; Schmidt, Anatol; Schreiner, Matthias; Mayer, Helmut K. (2019). "Green coffee infusion as a source of caffeine and chlorogenic acid". Journal of Food Composition and Analysis. 84: 103307. doi:10.1016/j.jfca.2019.103307. S2CID 202882087.
- ^ Naveed, M; Hejazi, V; Abbas, M; Kamboh, AA; Khan, GJ; Shumzaid, M; Ahmad, F; Babazadeh, D; FangFang, X; Modarresi-Ghazani, F; WenHua, L; XiaoHui, Z (January 2018). "Chlorogenic acid (CGA): A pharmacological review and call for further research". Biomedicine & Pharmacotherapy. 97: 67–74. doi:10.1016/j.biopha.2017.10.064. PMID 29080460. Retrieved 10 September 2020.
- ^ Tajik, N; Tajik, M; Mack, I; Enck, P (2017). "The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature". European Journal of Nutrition. 56 (7): 2215–2244. doi:10.1007/s00394-017-1379-1. PMID 28391515. S2CID 5177390.
- ^ Onakpoya, I. J.; Spencer, E. A.; Thompson, M. J.; Heneghan, C. J. (19 June 2014). "The effect of chlorogenic acid on blood pressure: a systematic review and meta-analysis of randomized clinical trials". Journal of Human Hypertension. 29 (2): 77–81. doi:10.1038/jhh.2014.46. PMID 24943289. S2CID 2881228.
- ^ "Substances generally recognized as safe". Code of Federal Regulations, Title 21, Part 582, US Food and Drug Administration. 20 April 2022. Retrieved 25 April 2022.
- ^ "Green coffee". MedlinePlus, US National Library of Medicine. 11 August 2021. Retrieved 25 April 2022.
- ^ Ventura, K. (2016). "Unremitting problems with chlorogenic acid nomenclature: a review". Química Nova. 39 (4): 530–533. doi:10.5935/0100-4042.20160063. hdl:10195/66766.
- ^ M. N. Clifford and L. Abranko. Some Notes on the Chlorogenic Acids. 1. Numbering and Nomenclature. ResearchGate. doi:10.13140/RG.2.2.22301.31202 2017.

