Alendronic acid
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Fosamax, Binosto, others |
| Other names | Alendronate, alendronate sodium (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601011 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 0.6% |
| Metabolism | excreted unchanged |
| Elimination half-life | 126 months |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.128.415 |
| Chemical and physical data | |
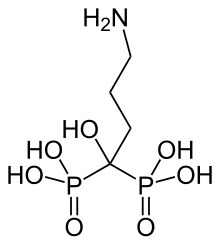
| Formula | C4H13NO7P2 |
| Molar mass | 249.096 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Alendronic acid, sold under the brand name Fosamax among others, is a bisphosphonate medication used to treat osteoporosis and Paget's disease of bone.[4] It is taken by mouth.[4] Use is often recommended together with vitamin D, calcium supplementation, and lifestyle changes.[4]
Common side effects (1 to 10% of patients) include constipation, abdominal pain, nausea, and acid reflux.[4] Use is not recommended during pregnancy or in those with poor kidney function.[5] Alendronic acid works by decreasing the activity of cells that break down bone.[4]
Alendronic acid was first described in 1978 and approved for medical use in the United States in 1995.[4][6] It is available as a generic medication. In 2022, it was the 103rd most commonly prescribed medication in the United States, with more than 6 million prescriptions.[7][8]
Medical uses
[edit]Alendronic acid is indicated for the treatment and prevention of osteoporosis in postmenopausal women;[3] the treatment to increase bone mass in men with osteoporosis;[3] the treatment of glucocorticoid-induced osteoporosis;[3] and the treatment of Paget's disease of bone.[3][4]
Side effects
[edit]- Gastrointestinal tract:
- Ulceration and possible rupture of the esophagus; this may require hospitalization and intensive treatment. Gastric and duodenal ulceration may also occur.
- Esophageal cancer, a meta-analysis concluded that bisphosphonate treatment is NOT associated with excess risk of esophageal cancer.[9][10]
- General: infrequent cases of skin rash, rarely manifesting as Stevens–Johnson syndrome and toxic epidermal necrolysis, eye problems (uveitis, scleritis) and generalized muscle, joint, and bone pain[11] (rarely severe) have been reported.
- Osteonecrosis of the jaw may occur while on this drug, if dental work of any kind is carried out. The risk is considerably higher for extractions in the mandible (lower jaw) than other areas of the mouth, and the risk increases if you have been taking it for four or more years [12] Although this side effect is uncommon (0.4-1.6% for oral alendronic acid), it occurs primarily in patients being administered intravenous bisphosphonates, with most cases being reported in cancer patients.[13][14]
- Bone: alendronate has been linked in long-term users to the development of low-impact femoral fractures.[15] Further, studies suggest that users of alendronate have an increase in the numbers of osteoclasts and develop giant, more multinucleated osteoclasts; the significance of this development is unclear.[16] Fosamax has been linked to a rare type of leg fracture that cuts straight across the upper thigh bone after little or no trauma (subtrochanteric fractures).[17]
Pharmacology
[edit]Mechanism of action
[edit]Nitrogen containing bisphosphonates, which include ibandronate, pamidronate and alendronate exert their effects on osteoclasts mainly by inhibiting the synthesis of isoprenoid lipids such as isopentenyl diphosphate (IPP), farnesyl diphosphate (FPP), and geranylgeranyl diphosphate (GGPP) via the mevalonate pathway. These isoprenoids are used in posttranslational modifcation(prenylation) of small GTPases such as Ras, Rho, and Rac. These prenylated GTPases are necessary for various cellular processes including osteoclast morphology, endosome trafficking, and apoptosis. Alendronate has also been shown to impair the function of osteclast lysosomes.[18]
| Bisphosphonate | Relative potency |
|---|---|
| Etidronate | 1 |
| Tiludronate | 10 |
| Pamidronate | 100 |
| Alendronate | 100-500 |
| Ibandronate | 500-1000 |
| Risedronate | 1000 |
| Zoledronate | 5000 |
Pharmacokinetics
[edit]The fraction of the drug that reaches the circulatory system intact (systemic bioavailability) after oral dosing is low, averaging only 0.6–0.7% in women and in men under fasting conditions. Intake together with meals and beverages other than water further reduces the bioavailability. The absorbed drug rapidly partitions, with approximately 50% binding to the exposed bone surface; the remainder is excreted unchanged by the kidneys. Unlike with most drugs, the strong negative charge on the two phosphonate moieties limits oral bioavailability, and, in turn, the exposure to tissues other than bone is very low. After absorption in the bone, alendronate has an estimated terminal elimination half-life of 10 years.[20]
References
[edit]- ^ "Alendronate Use During Pregnancy". Drugs.com. 22 August 2019. Retrieved 17 May 2020.
- ^ "Product monograph brand safety updates". Health Canada. 7 July 2016. Retrieved 3 April 2024.
- ^ a b c d e "Fosamax- alendronate sodium tablet". DailyMed. 16 April 2024. Retrieved 30 September 2024.
- ^ a b c d e f g "Fosamax Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 2 February 2019.
- ^ British National Formulary : BNF 76 (76 ed.). Pharmaceutical Pres s. 2018. pp. 710–711. ISBN 978-0-85711-338-2.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 523. ISBN 978-3-527-60749-5.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Alendronate Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ Sun K, Liu JM, Sun HX, Lu N, Ning G (January 2013). "Bisphosphonate treatment and risk of esophageal cancer: a meta-analysis of observational studies". Osteoporosis International. 24 (1): 279–286. doi:10.1007/s00198-012-2158-8. PMID 23052941. S2CID 12625687.
- ^ Haber SL, McNatty D (March 2012). "An evaluation of the use of oral bisphosphonates and risk of esophageal cancer". The Annals of Pharmacotherapy. 46 (3): 419–423. doi:10.1345/aph.1Q482. PMID 22333262. S2CID 38417272.
- ^ "Severe Pain with Osteoporosis Drugs". FDA Patient Safety News. March 2008. Archived from the original on 15 April 2014.
- ^ "Fosamax product description" (PDF). Merck & Co.
- ^ Pazianas M, Miller P, Blumentals WA, Bernal M, Kothawala P (August 2007). "A review of the literature on osteonecrosis of the jaw in patients with osteoporosis treated with oral bisphosphonates: prevalence, risk factors, and clinical characteristics". Clinical Therapeutics. 29 (8): 1548–1558. doi:10.1016/j.clinthera.2007.08.008. PMID 17919538.
- ^ Carini F, Barbano L, Saggese V, Monai D, Porcaro G (April 2012). "Multiple systemic diseases complicated by bisphosphonate osteonecrosis: a case report". Annali di Stomatologia. 3 (2 Suppl): 32–36. PMC 3512552. PMID 23285320.
- ^ Lenart BA, Lorich DG, Lane JM (March 2008). "Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate". The New England Journal of Medicine. 358 (12): 1304–1306. doi:10.1056/NEJMc0707493. PMID 18354114. S2CID 26968573.
- Lay summary in: Gordon S (19 March 2008). "Fosamax Linked to Unusual Femur Fractures". U.S. News & World Report. Archived from the original on 16 April 2010.
- ^ Weinstein RS, Roberson PK, Manolagas SC (January 2009). "Giant osteoclast formation and long-term oral bisphosphonate therapy". The New England Journal of Medicine. 360 (1): 53–62. doi:10.1056/NEJMoa0802633. PMC 2866022. PMID 19118304.
- Lay summary in: Gardner A (31 December 2008). "Osteoporosis Drug Prompts Increase in Certain Bone Cells". Washington Post.
- ^ Kwek EB, Goh SK, Koh JS, Png MA, Howe TS (February 2008). "An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy?". Injury. 39 (2): 224–231. doi:10.1016/j.injury.2007.08.036. PMID 18222447.
- ^ Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, et al. (June 2000). "Cellular and molecular mechanisms of action of bisphosphonates". Cancer. 88 (12 Suppl): 2961–2978. doi:10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. PMID 10898340.
- ^ Tripathi DK (30 September 2013). Essentials of medical pharmacology (Seventh ed.). New Delhi. ISBN 978-9-350-25937-5. OCLC 868299888.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Shinkai I, Ohta Y (January 1996). "New drugs--reports of new drugs recently approved by the FDA. Alendronate". Bioorganic & Medicinal Chemistry. 4 (1): 3–4. doi:10.1016/0968-0896(96)00042-9. PMID 8689235.
