Melissic acid
Appearance
(Redirected from C30H60O2)
| |
| Names | |
|---|---|
| Preferred IUPAC name
Triacontanoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.312 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H60O2 | |
| Molar mass | 452.46 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
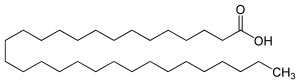
Melissic acid (or triacontanoic acid) is the organic compound with the formula CH3(CH2)28CO2H. It is classified as a very long chain fatty acid, a subset of saturated fatty acids. It is a white solid that is soluble in organic solvents. Melissic acid gets its name from the Greek word melissa meaning bee, since it was found in beeswax.
Synthesis
[edit]n-Triacontanoic acid was synthesized by Bleyberg and Ulrich (1931) and by G.M. Robinson.[1]
Self-assembly
[edit]Triacontanoic acid and triacontanamide (CH3(CH2)28-CONH2) can be self-assembled.[2]
See also
[edit]References
[edit]- ^ Chibnall, Albert Charles; Ernest Frank Williams; Alfred Louis Latner; Stephen Harvey Piper (1933). "The isolation of n-triacontanol from lucerne wax". Biochemical Journal. 27 (6): 1885–1888. doi:10.1042/bj0271885. PMC 1253114. PMID 16745314.
- ^ Weinbach, Susan P.; Kristian Kjaer; Jens Als-Nielsen; Meir Lahav; Leslie Leiserowitz (May 1993). "Self-assembled Langmuir monolayers and trilayers at the air-formamide interface". Journal of Physical Chemistry. 97 (20): 5200–5203. doi:10.1021/j100122a003.
External links
[edit]- Melissic acid at the Nature Lipidomics Gateway
