Oxathiazolones
 1,3,4-Oxathiazol-2-one
| |
| Names | |
|---|---|
| Preferred IUPAC name
2H-1,3,4-Oxathiazol-2-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C2HNO2S | |
| Molar mass | 103.10 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The oxathiazolones are a family of heterocyclic compounds in which the parent derivative has the molecular formula C2HNO2S and for which multiple isomers are known. The two known isomers with the highest profile in the literature are 1,3,4-oxathiazol-2-one[1] and 1,4,2-oxathiazol-5-one.[2]

1,3,4-Oxathiazol-2-one
[edit]Molecular and electronic structure
[edit]1,3,4-Oxathiaol-2-one derivatives are planar heterocycles that prefer co-planarity with aromatic substituents.[3] It has been proposed that the π system of the ring consists of CNS and CO2 "π islands" that prefer coplanarity to enhance inter-ring π conjugation.[3]
Synthesis
[edit]The traditional route for 1,3,4-oxathiazol-2-one synthesis is via 1,3 dipolar cycloaddition, where chlorocarbonylsulfenyl chloride and amide are heated together in an appropriate solvent.[1] Appropriate solvents must dissolve the amide. Typically toluene or chloroform is used. A wide variety of amides have been used is the synthesis of 1,3,4-oxathiazol-2-one yielding various derivatives. Variations in this procedure have included doing the reaction under an inert atmosphere, adding chlorocarbonylsulfenyl chloride drop-wise, and varying the ratio of chlorocarbonylsulfenyl chloride to amide. Variations in procedure may be due to local preferences or substituent effects.[citation needed]
Reactions
[edit]Decarboxylation leading to isothiazole derivatives
[edit]1,3,4-Oxathiazol-2-one derivatives are commonly used in thermal decarboxylation reactions to generate the corresponding derivative of the short-lived nitrile sulfide which may be trapped by 1,3-dipolar cycloaddition reactions to give heterocycles in low to high yields depending on the nature of the substituent groups.[4]

The intermediate can be trapped with a suitable electron deficient dipolariphile to give stable heterocycles such as isothiazole (seen below).

Other decarboxylation reactions
[edit]The intermediate has been successfully trapped using other dipolarophiles including nitriles, alkenes, and phosphaalkenes.
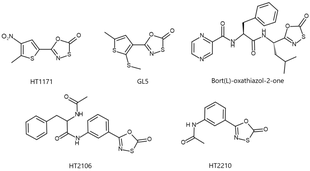
Biological significance and applications
[edit]Some 1,3,4-oxathiazol-2-one heterocycles have demonstrated selective inhibition of proteasomes in Mycobacterum tuberculosis and humans. Oxathiazolones HT1171 and GL5 (right) selectively inhibited the M. tuberculosis 26S proteasome and were over 1000-fold less effective on the human proteasome even in high concentrations.[5] Various 5‐styryl‐oxathiazol‐2‐one heterocycles have also been tested as anti-tubercular agents because of their ability to inhibit the M. tuberculosis 26S proteasome.[8]
A Bortezomib derived 1,3,4-oxathiazol-2-one (bort(L)-oxathiazol-2-one, right) selectively acts against the human proteasome rather than bacterial proteasomes, much like Bortezomib.[6] HT2210 and HT2106 (right) were found to have similar effects.[7] Human proteasome inhibition is useful in the treatment of cancer, neurodegenerative disorders, and inflammation.[9]
See also
[edit]- Oxazolone, an analog without the sulfur atom.
- Thiazole and isothiazole, analogues without the carbonyl group or oxygen atom.
- Oxazole and isoxazole, analogues without the carbonyl group or sulfur atom.
References
[edit]- ^ a b Jukič M, Grabrijan K, Kadić S, Lera Garrido FJ, Sosič I, Gobec S, Obreza A (December 2017). "Chlorocarbonylsulfenyl Chloride Cyclizations Towards Piperidin-3-yl-oxathiazol-2-ones as Potential Covalent Inhibitors of Threonine Proteases". Acta Chimica Slovenica. 64 (4): 771–781. doi:10.17344/acsi.2017.3883. PMID 29318298.
- ^ Argyropoulos NG (1996-01-01). "4.14 - 1,4-Oxa/thia-2-azoles". In Katritzky AR, Rees CW, Scriven EF (eds.). Comprehensive Heterocyclic Chemistry II. Oxford: Pergamon. pp. 491–543. ISBN 978-0-08-096518-5. Retrieved 2020-10-11., and references therein.
- ^ a b Krayushkin MM, Kalik MA, Vorontsova LG (2010-08-01). "The effect of the nature of the substituent on the structure of a 1,3,4-oxathiazol-2-one ring". Chemistry of Heterocyclic Compounds. 46 (4): 484–489. doi:10.1007/s10593-010-0535-9. S2CID 55033194.
- ^ Marion C. McKie and R. Michael Paton (2002). "Synthesis of 5-acyl-1,2,4-thiadiazoles by cycloaddition of nitrile sulfides to acylcyanides". Arkivoc (vi): 15–21.
- ^ a b Lin G, Li D, de Carvalho LP, Deng H, Tao H, Vogt G, et al. (October 2009). "Inhibitors selective for mycobacterial versus human proteasomes". Nature. 461 (7264): 621–6. Bibcode:2009Natur.461..621L. doi:10.1038/nature08357. PMC 3172082. PMID 19759536.
- ^ a b Gryder BE, Guerrant W, Chen CH, Oyelere AK (2011). "Oxathiazole-2-one derivative of bortezomib: Synthesis, stability and proteasome inhibition activity". MedChemComm. 2 (11): 1083–1086. doi:10.1039/C1MD00208B.
- ^ a b Fan H, Angelo NG, Warren JD, Nathan CF, Lin G (April 2014). "Oxathiazolones Selectively Inhibit the Human Immunoproteasome over the Constitutive Proteasome". ACS Medicinal Chemistry Letters. 5 (4): 405–10. doi:10.1021/ml400531d. PMC 4027612. PMID 24900849.
- ^ Russo F, Gising J, Åkerbladh L, Roos AK, Naworyta A, Mowbray SL, et al. (June 2015). "Optimization and Evaluation of 5-Styryl-Oxathiazol-2-one Mycobacterium tuberculosis Proteasome Inhibitors as Potential Antitubercular Agents". ChemistryOpen. 4 (3): 342–62. doi:10.1002/open.201500001. PMC 4522185. PMID 26246997.
- ^ de Bettignies G, Coux O (November 2010). "Proteasome inhibitors: Dozens of molecules and still counting". Biochimie. 92 (11): 1530–45. doi:10.1016/j.biochi.2010.06.023. PMID 20615448.
