Ruboxistaurin
Appearance
(Redirected from C28H28N4O3)
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
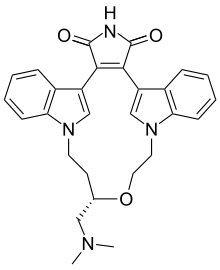
| Formula | C28H28N4O3 |
| Molar mass | 468.557 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ruboxistaurin (proposed brand name Arxxant) is an investigational drug for diabetic retinopathy being investigated by Eli Lilly and Company. It is a member of the bisindolylmaleimide family.
In February 2006, Lilly submitted a New Drug Application for ruboxistaurin, and on August 18, 2006, Lilly received an approvable letter from the US FDA for ruboxistaurin,[1] with a request for an additional clinical trial, which would take 5 years to complete.[2] Lilly has not made any further request for approval and ruboxistaurin is not approved by the FDA for any medical use.[3]
Mechanism of action
[edit]Ruboxistaurin is an inhibitor of protein kinase C-beta.[4]
References
[edit]- ^ "Drugs.com, Eli Lilly and Company Announces Approvable Letter Issued by FDA for Arxxant". Retrieved 2008-02-15.
- ^ "Drugs.com, Lilly Announces FDA Requirement of Additional Clinical Trial Before Ruboxistaurin Could Be Approved for Treatment of Diabetic Retinopathy". Retrieved 2008-02-15.
- ^ "Arxxant Approval Status". drugs.com.
- ^ Clarke M, Dodson PM (December 2007). "PKC inhibition and diabetic microvascular complications". Best Pract Res Clin Endocrinol Metab. 21 (4): 573–86. doi:10.1016/j.beem.2007.09.007. PMID 18054736.
External links
[edit]
