Nimodipine
 | |
| Clinical data | |
|---|---|
| Trade names | Nimotop, Nymalize, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a689010 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, by mouth |
| Drug class | Dihydropyridine calcium channel blocker |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 13% (by mouth) |
| Protein binding | 95% |
| Metabolism | Hepatic |
| Elimination half-life | 8–9 hours |
| Excretion | Feces and Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.060.096 |
| Chemical and physical data | |
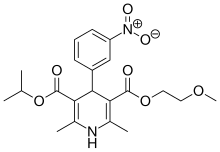
| Formula | C21H26N2O7 |
| Molar mass | 418.446 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 125 °C (257 °F) |
| |
| |
| (verify) | |
Nimodipine, sold under the brand name Nimotop among others, is a calcium channel blocker used in preventing vasospasm secondary to subarachnoid hemorrhage (a form of cerebral hemorrhage). It was originally developed within the calcium channel blocker class as it was used for the treatment of high blood pressure, but is not used for this indication.
It was patented in 1971[3] and approved for medical use in the US in 1988.[4] It was approved for medical use in Germany in 1985.[5]
Medical use
[edit]Because it has some selectivity for cerebral vasculature, nimodipine's main use is in the prevention of cerebral vasospasm and resultant ischemia, a complication of subarachnoid hemorrhage (a form of cerebral bleed), specifically from ruptured intracranial berry aneurysms irrespective of the patient's post-ictus neurological condition.[6] Its administration begins within 4 days of a subarachnoid hemorrhage and is continued for three weeks. If blood pressure drops by over 5%, dosage is adjusted. There is still controversy regarding the use of intravenous nimodipine on a routine basis.[7][8]
A 2003 trial (Belfort et al.) found nimodipine was inferior to magnesium sulfate in preventing seizures in women with severe preeclampsia.[9]
Nimodipine is not regularly used to treat head injury. Several investigations have been performed evaluating its use for traumatic subarachnoid hemorrhage; a systematic review of 4 trials did not suggest any significant benefit to the patients that receive nimodipine therapy.[10] There was one report case of nimodipine being successfully used for treatment of ultradian bipolar cycling after brain injury and, later, amygdalohippocampectomy.[11]
Dosage
[edit]The regular dosage is 60 mg tablets every four hours. If the patient is unable to take tablets orally, it was previously given via intravenous infusion at a rate of 1–2 mg/hour (lower dosage if the body weight is <70 kg or blood pressure is too low),[7] but since the withdrawal of the IV preparation, administration by nasogastric tube is an alternative.
Contraindications
[edit]Nimodipine is associated with low blood pressure, flushing and sweating, edema, nausea and other gastrointestinal problems, most of which are known characteristics of calcium channel blockers. It is contraindicated in unstable angina or an episode of myocardial infarction more recently than one month.[citation needed]
While nimodipine was occasionally administered intravenously in the past, the FDA released an alert in January 2006, warning that it had received reports of the approved oral preparation being used intravenously, leading to severe complications; this was despite warnings on the box that this should not be done.[12]
Side-effects
[edit]The FDA has classified the side effects into groups based on dosages levels at q4h. For the high dosage group (90 mg) less than 1% of the group experienced adverse conditions including itching, gastrointestinal hemorrhage, thrombocytopenia, neurological deterioration, vomiting, diaphoresis, congestive heart failure, hyponatremia, decreasing platelet count, disseminated intravascular coagulation, deep vein thrombosis.[6]
Pharmacokinetics
[edit]Absorption
[edit]After oral administration, it reaches peak plasma concentrations within one and a half hours. Patients taking enzyme-inducing anticonvulsants have lower plasma concentrations, while patients taking sodium valproate were markedly higher.[13]
Metabolism
[edit]Nimodipine is metabolized in the first pass metabolism. The dihydropyridine ring of the nimodipine is dehydrogenated in the hepatic cells of the liver, a process governed by cytochrome P450 isoform 3A (CYP3A). This can be completely inhibited however, by troleandomycin (an antibiotic) or ketoconazole (an antifungal drug).[14]
Excretion
[edit]Studies in non-human mammals using radioactive labeling have found that 40–50% of the dose is excreted via urine. The residue level in the body was never more than 1.5% in monkeys.[citation needed]
Mode of action
[edit]Nimodipine binds specifically to L-type voltage-gated calcium channels. There are numerous theories about its mechanism in preventing vasospasm, but none are conclusive.[15]
Nimodipine has additionally been found to act as an antagonist of the mineralocorticoid receptor, or as an antimineralocorticoid.[16]
Synthesis
[edit]
The key acetoacetate (2) for the synthesis of nimodipine (5) is obtained by alkylation of sodium acetoacetate with 2-methoxyethyl chloride, Aldol condensation of meta-nitrobenzene (1) and the subsequent reaction of the intermediate with enamine (4) gives nimodipine.
Stereochemistry
[edit]Nimodipine contains a stereocenter and can exist as either of two enantiomers. The pharmaceutical drug is a racemate, an equal mixture of the (R)- and (S)- forms.[18]
| Enantiomers of nimodipine | |
|---|---|
 (R)-Nimodipine CAS number: 77940-92-2 |
 (S)-Nimodipine CAS number: 77940-93-3 |
References
[edit]- ^ "Nimodipine Use During Pregnancy". Drugs.com. March 15, 2019. Retrieved April 11, 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved October 22, 2023.
- ^ GB 1358951, Meyer H, Bossert F, Vater W, Stoepel KN, "New esters, their production, and their medicinal use", published 1974-07-03, assigned to Bayer AG
- ^ "US FDA NDA 018869" (New drug approval from the US FDA). Drugs@FDA.gov Approved Drugs. Food and Drug Administration of the United States (FDA). December 28, 1988. Retrieved April 11, 2019.
Nimodipine (...) approved for the treatment of high blood pressure (...)
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 464. ISBN 9783527607495.
- ^ a b "FDA approved Labeling text. Nimotop (nimodipine) Capsules For Oral Use" (PDF). Food and Drug Administration. December 2005. Retrieved July 21, 2009.
- ^ a b Janjua N, Mayer SA (April 2003). "Cerebral vasospasm after subarachnoid hemorrhage". Current Opinion in Critical Care. 9 (2): 113–119. doi:10.1097/00075198-200304000-00006. PMID 12657973. S2CID 495267.
- ^ Allen GS, Ahn HS, Preziosi TJ, Battye R, Boone SC, Boone SC, et al. (March 1983). "Cerebral arterial spasm--a controlled trial of nimodipine in patients with subarachnoid hemorrhage". The New England Journal of Medicine. 308 (11): 619–624. doi:10.1056/NEJM198303173081103. PMID 6338383.
- ^ Belfort MA, Anthony J, Saade GR, Allen JC (January 2003). "A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia". The New England Journal of Medicine. 348 (4): 304–311. doi:10.1056/NEJMoa021180. PMID 12540643.
- ^ Vergouwen MD, Vermeulen M, Roos YB (December 2006). "Effect of nimodipine on outcome in patients with traumatic subarachnoid haemorrhage: a systematic review". The Lancet. Neurology. 5 (12): 1029–1032. doi:10.1016/S1474-4422(06)70582-8. PMID 17110283. S2CID 43488740.
- ^ De León OA (February 2012). "Response to nimodipine in ultradian bipolar cycling after amygdalohippocampectomy". Journal of Clinical Psychopharmacology. 32 (1): 146–148. doi:10.1097/JCP.0b013e31823f9116. PMID 22217956.
- ^ "Information for Healthcare Professionals: Nimodipine (marketed as Nimotop)". Food and Drug Administration. Archived from the original on July 22, 2017. Retrieved July 21, 2009.
- ^ Tartara A, Galimberti CA, Manni R, Parietti L, Zucca C, Baasch H, et al. (September 1991). "Differential effects of valproic acid and enzyme-inducing anticonvulsants on nimodipine pharmacokinetics in epileptic patients". British Journal of Clinical Pharmacology. 32 (3): 335–340. doi:10.1111/j.1365-2125.1991.tb03908.x. PMC 1368527. PMID 1777370.
- ^ Liu XQ, Ren YL, Qian ZY, Wang GJ (August 2000). "Enzyme kinetics and inhibition of nimodipine metabolism in human liver microsomes" (PDF). Acta Pharmacologica Sinica. 21 (8): 690–694. PMID 11501176. Archived from the original (PDF) on July 8, 2011. Retrieved April 11, 2009.
- ^ Rang HP (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4.
- ^ Luther JM (September 2014). "Is there a new dawn for selective mineralocorticoid receptor antagonism?". Current Opinion in Nephrology and Hypertension. 23 (5): 456–461. doi:10.1097/MNH.0000000000000051. PMC 4248353. PMID 24992570.
- ^ DE 2117571, Meyer H, Bossert F, Vater W, Stoepel KN, "Unsymmetrische 1,4-Dihydropyridincarbonsäureester, Verfahren zu ihrer Herstellung sowie ihre Verwendung als Arzneimitell I [Asymmetrical 1,4-dihydropyridine carboxylic acid esters, process for their preparation and their use as pharmaceuticals I]", published 1972-10-19, assigned to Bayer AG
- ^ Rote Liste Service GmbH (Hrsg.): Rote Liste 2017 – Arzneimittelverzeichnis für Deutschland (einschließlich EU-Zulassungen und bestimmter Medizinprodukte). Rote Liste Service GmbH, Frankfurt/Main, 2017, Aufl. 57, ISBN 978-3-946057-10-9, S. 204.
Further reading
[edit]- Towart R, Kazda S (November 1979). "The cellular mechanism of action of nimodipine (BAY e 9736), a new calcium antagonist [proceedings]". British Journal of Pharmacology. 67 (3): 409P–410P. doi:10.1111/j.1476-5381.1979.tb08695.x. PMC 2044020. PMID 497542.
- Deyo RA, Straube KT, Disterhoft JF (February 1989). "Nimodipine facilitates associative learning in aging rabbits". Science. 243 (4892). New York, N.Y.: 809–11. Bibcode:1989Sci...243..809D. doi:10.1126/science.2916127. PMID 2916127.
- Use as cerebral vasodilator: GB 2018134, "Cerebral therapeutic agent", assigned to Bayer AG; eidem, US 4406906, Meyer H, Bossert F, Kazda S, Hoffmeister F, Vater W, issued September 27, 1983, assigned to Bayer AG
