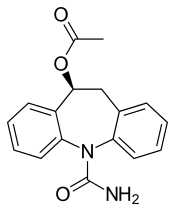
Eslicarbazepine acetate
 | |
| Clinical data | |
|---|---|
| Trade names | Aptiom, Zebinix, Exalief |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | ~30%[5] |
| Metabolism | UGT (?) |
| Metabolites | Eslicarbazepine (active), glucuronides (inactive), etc. |
| Elimination half-life | 10–20 hours |
| Excretion | ~90% renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.398 |
| Chemical and physical data | |
| Formula | C17H16N2O3 |
| Molar mass | 296.326 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Eslicarbazepine acetate (ESL), sold under the brand names Aptiom and Zebinix among others, is an anticonvulsant medication approved for use in Europe and the United States as monotherapy or as additional therapy for partial-onset seizures epilepsy.[6][4][3]
Similarly to oxcarbazepine, ESL behaves as a prodrug to (S)-(+)-licarbazepine.[7] As such, their mechanisms of action are identical.[8]
Contraindications
[edit]Eslicarbazepine acetate is contraindicated in people with second- or third-degree atrioventricular block, a type of heart block, and in people who are hypersensitive to eslicarbazepine, oxcarbazepine or carbamazepine.[9]
Adverse effects
[edit]Adverse effects are similar to oxcarbazepine. The most common ones (more than 10% of patients) are tiredness and dizziness. Other fairly common side effects (1 to 10%) include impaired coordination, gastrointestinal disorders such as diarrhoea, nausea and vomiting, rash (1.1%), and hyponatremia (low sodium blood levels, 1.2%).[3][9] There may also be an increased risk of suicidal thoughts.[10]
Overdose
[edit]Symptoms of overdosing are similar to adverse effects of standard doses: severe hyponatraemia, somnolence, uncoordinated/unsteady gait, hemiparesis (weakness of one side of the body), along with visual and gastrointestinal disturbances. No specific antidote is available. Eslicarbazepine and metabolites can be dialyzed.[3][9]
Interactions
[edit]Like oxcarbazepine, eslicarbazepine can reduce plasma levels of drugs that are metabolized by the liver enzymes CYP3A4 (verified in studies for simvastatin and the oral contraceptive levonorgestrel/ethinylestradiol) and UDP-glucuronosyltransferase, and increase plasma levels of drugs metabolized by CYP2C19.[3][9]
Interaction studies have been conducted with a number of common anticonvulsants. Carbamazepine reduces blood plasma concentrations of eslicarbazepine, probably because it induces glucuronidation. This drug combination also increased the risk for diplopia, impaired coordination and dizziness in a clinical study. Phenytoin also reduces eslicarbazepine plasma concentrations, which may be due to increased glucuronidation of eslicarbazepine; and concomitant administration results in an increase in phenytoin serum concentrations, which is probably due to inhibition of CYP2C19.[10] Combinations with lamotrigine, topiramate, valproic acid or levetiracetam showed no significant interactions in studies, although eslicarbazepine has been shown to cause a minor reduction in lamotrigine levels.[9][10]
Pharmacology
[edit]Mechanism of action
[edit]The active component, eslicarbazepine, has the same mechanism of action as oxcarbazepine (which is a prodrug for licarbazepine, the racemate of eslicarbazepine) and most likely the closely related carbamazepine. It stabilises the inactive state of voltage-gated sodium channels, allowing for less sodium to enter neural cells, which leaves them less excitable.[11] According to some sources, it has not been shown conclusively that this is the actual mechanism.[3][9]
Pharmacokinetics
[edit]Eslicarbazepine acetate is absorbed to at least 90% from the gut, independently of food intake. It is quickly metabolised to eslicarbazepine, so that the original substance cannot be detected in the bloodstream. Peak plasma levels of eslicarbazepine are reached after 2–3 (1–4) hours, and plasma protein binding is somewhat less than 40%. Biological half-life is 10 to 20 hours, and steady-state concentrations are reached after four to five days after start of the treatment.[3][9] Oxcarbazepine, for comparison, is also nearly completely absorbed from the gut, and peak plasma concentrations of licarbazepine are reached after 4.5 hours on average after oxcarbazepine intake. Plasma protein binding and half-life are of course the same.[12]
Other metabolites of ESL are the less active (R)-(−)-licarbazepine (5%; the stereoisomer of eslicarbazepine), the pharmacologically active oxcarbazepine (1%), and inactive glucuronides of all of these substances. The drug is excreted mainly via the urine, of which two thirds are in the form of eslicarbazepine and one third in the form of eslicarbazepine glucuronide. The other metabolites only account for a few percent of the excreted drug.[3][9]
Pharmacogenomics
[edit]Persons with certain genetic variations in human leukocyte antigens (HLAs) are under increased risk of developing skin reactions such as acute generalized exanthematous pustulosis (AGEP), but also severe ones such as Stevens–Johnson and DRESS syndrome, under treatment with carbamazepine and drugs with related chemical structures. This is true for the HLA-A*3101 allele, which occurs in 2 to 5% of Europeans and 10% of Japanese people, and the HLA-B*1502 allele, which is mainly found in people of Asian descent. Theoretically, this may also apply to ESL.[9]
Chemistry
[edit]As the name suggests, eslicarbazepine acetate is the acetate ester prodrug of eslicarbazepine. Eslicarbazepine itself is the pharmacologically more active of the two stereoisomers of licarbazepine.[3] More specifically, it is (S)-(+)-licarbazepine.
- Related drugs and active metabolites for comparison
-
Eslicarbazepine, the active metabolite of ESL
-
Licarbazepine, the active metabolite of oxcarbazepine
History
[edit]Eslicarbazepine acetate was developed by the Portuguese pharmaceutical company Bial. In early 2009, Bial sold the marketing rights in Europe to the Japanese company Eisai.[13] The drug was approved in the European Union in April 2009 under the trade names Zebinix and Exalief, but was marketed only under the first name.[14][15] In the US it is marketed by Sunovion (formerly Sepracor) and was approved in November 2013.[6]
Research
[edit]Studies for the use of ESL as an anticonvulsant for children are under way as of 2016[update].[16]
Like oxcarbazepine, ESL has potential uses for the treatment of trigeminal neuralgia[citation needed] and bipolar disorder. A 2015 assessment showed no statistical difference to placebo for the latter disorder.[17]
References
[edit]- ^ a b "Zebinix". Therapeutic Goods Administration (TGA). 9 June 2021. Retrieved 6 September 2021.
- ^ "Summary Basis of Decision (SBD) for Aptiom". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ a b c d e f g h i "Aptiom- eslicarbazepine acetate tablet Aptiom- eslicarbazepine acetate kit". DailyMed. Retrieved 21 January 2021.
- ^ a b "Zebinix EPAR". European Medicines Agency (EMA). Retrieved 21 January 2021.
- ^ Dinnendahl V, Fricke U, eds. (2011). Arzneistoff-Profile (in German). Vol. 4 (25 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- ^ a b "FDA approves Aptiom to treat seizures in adults". US FDA. 8 November 2013. Archived from the original on 11 January 2017. Retrieved 16 December 2019.
- ^ Rogawski MA (June 2006). "Diverse mechanisms of antiepileptic drugs in the development pipeline". Epilepsy Research. 69 (3): 273–94. doi:10.1016/j.eplepsyres.2006.02.004. PMC 1562526. PMID 16621450.
- ^ Rogawski MA, Löscher W (July 2004). "The neurobiology of antiepileptic drugs". Nature Reviews. Neuroscience. 5 (7): 553–64. doi:10.1038/nrn1430. PMID 15208697. S2CID 2201038.
- ^ a b c d e f g h i Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. 2015. Zebinix.
- ^ a b c "Zebinix 800mg tablets". Electronic Medicines Compendium (eMC). Retrieved 12 April 2017.
- ^ Almeida L, Soares-da-Silva P (January 2007). "Eslicarbazepine acetate (BIA 2-093)". Neurotherapeutics. 4 (1): 88–96. doi:10.1016/j.nurt.2006.10.005. PMC 7479690. PMID 17199020.
- ^ Jasek W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. p. 8384. ISBN 978-3-85200-181-4.
- ^ "Eisai and Bial Announce Partnership Agreement for the European Commercialisation of the Novel Once Daily Anti-Epileptic Zebinix". PR Newswire. 19 February 2009.
- ^ "Summary of Product Characteristics for Zebinix" (PDF). European Medicines Agency. p. 14. Archived from the original (PDF) on 2018-09-20. Retrieved 2016-05-22.
- ^ "Exalief (eslicarbazepine acetate): Expiry of the marketing authorisation in the European Union" (PDF). European Medicines Agency. 30 July 2012. Archived from the original (PDF) on 20 September 2018. Retrieved 22 May 2016.
- ^ Clinical trial number NCT00988156 for "Eslicarbazepine Acetate (BIA 2 093) as Therapy for Refractory Partial Seizures in Children" at ClinicalTrials.gov
- ^ Grunze H, Kotlik E, Costa R, Nunes T, Falcão A, Almeida L, Soares-da-Silva P (March 2015). "Assessment of the efficacy and safety of eslicarbazepine acetate in acute mania and prevention of recurrence: experience from multicentre, double-blind, randomised phase II clinical studies in patients with bipolar disorder I". Journal of Affective Disorders. 174: 70–82. doi:10.1016/j.jad.2014.11.013. PMID 25484179.
External links
[edit]- "Eslicarbazepine acetate". Drug Information Portal. U.S. National Library of Medicine.




