Iproheptine
 | |
| Clinical data | |
|---|---|
| Trade names | Metron, Susat |
| Other names | N-Isopropyl-1,5-dimethylhexylamine; N-Isopropyloctodrine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.034.283 |
| Chemical and physical data | |
| Formula | C11H25N |
| Molar mass | 171.328 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
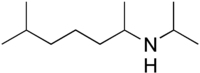
Iproheptine, also known as N-isopropyl-1,5-dimethylhexylamine or N-isopropyloctodrine and sold under the brand names Metron and Susat, is a nasal decongestant which has been marketed in Japan.[1][2][3] It is described as a vasoconstrictor and antihistamine.[1][2][3] The drug is available over-the-counter in Japan.[4]
Pharmacology
[edit]Pharmacodynamics
[edit]Iproheptine is described as a decongestant, vasoconstrictor, and antihistamine.[1][2][3] Its pharmacology was characterized in a series of several preclinical studies published in the 1960s.[5][6][7][8][9][10]
The drug was found to have anticholinergic- and antihistamine-like effects that were described as more potent than those of ephedrine.[6][8][10] It was said to have hypotensive and cardiac inhibitive actions that made it differ from other known alkylamine and arylalkylamine sympathomimetics.[6][8] The effects of iproheptine on blood vessels, pupils, and saliva secretion were all said to be very weak.[6] It produced bronchodilation, vasoconstriction, and hemostasis similarly to ephedrine or methoxyphenamine.[7][10] Iproheptine showed no effect against hexobarbital-induced sleep.[7] Conversely, it showed an antidepressant- or stimulant-like effect in the forced swim test (FST).[9]
Close analogues of iproheptine, such as methylhexanamine and tuaminoheptane, are known to act as norepinephrine and/or dopamine releasing agents by interacting with the monoamine transporters, and this is thought to underlie their sympathomimetic and stimulant effects.[11][12][13][14][15][16]
Pharmacokinetics
[edit]In contrast to arylalkylamines like phenethylamines and tryptamines, iproheptine is not metabolized by monoamine oxidase (MAO).[9]
Chemistry
[edit]Iproheptine, also known as N-isopropyl-1,5-dimethylhexylamine or as N-isopropyloctodrine, is an alkylamine and the N-isopropyl derivative of octodrine (2-amino-6-methylheptane or 1,5-dimethylhexylamine (1,5-DMHA)).[1][2][3]
Aside from octodrine, it is also closely structurally related to other alkylamines, including 1,3-dimethylbutylamine (1,3-DMBA), 1,4-dimethylamylamine (1,4-DMAA), heptaminol (2-methyl-6-amino-2-heptanol), isometheptene (2-methyl-6-methylamino-2-heptene), methylhexanamine (1,3-dimethylamylamine (1,3-DMAA)), and tuaminoheptane (tuamine; 2-aminoheptane or 1-methylhexylamine).[1][2][3]
Iproheptine shows structural similarity to what would be 3- or 4-methyl-N-isopropylamphetamine, but with the equivalent of the phenyl ring open and incomplete (i.e., missing two carbon atoms, saturated, and the carbons not connected to form a ring).[17][1]
The predicted log P (XLogP3) of iproheptine is 3.6.[17]
History
[edit]Iproheptine was first described in the scientific literature by 1960[5][6] and was first patented by 1962.[1] It remained marketed in Japan in 2004.[2]
Society and culture
[edit]Names
[edit]Iproheptine is the generic name of the drug and its INN.[1][2] In the case of the hydrochloride salt, its generic name is iproheptine hydrochloride and this is its JAN.[2] The drug is marketed under the brand names Metron and Susat (both as the hydrochloride salt).[1][2]
Availability
[edit]Iproheptine appears to have been marketed only in Japan.[2] It is available over-the-counter in this country.[4]
See also
[edit]References
[edit]- ^ a b c d e f g h i Elks J (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer US. p. 802. ISBN 978-1-4757-2085-3. Retrieved 30 August 2024.
- ^ a b c d e f g h i j Schweizerischer Apotheker-Verein (2004). Index Nominum: International Drug Directory. Index Nominum: International Drug Directory. Medpharm Scientific Publishers. p. 656. ISBN 978-3-88763-101-7. Retrieved 30 August 2024.
- ^ a b c d e Milne GW (2018). Drugs: Synonyms and Properties. Routledge Revivals. Taylor & Francis. p. 286. ISBN 978-1-351-78990-5. Retrieved 30 August 2024.
Iproheptine 13946-02-6 6243 CHAN N-Isopropyl-1,5-dimethylhexylamine. Metron; Metron S. Antihistaminic.
- ^ a b "KEGG DRUG: Iproheptine Hydrochloride". GenomeNet. Retrieved 30 August 2024.
- ^ a b Ota Y, Otani G, Enomoto R (1960). "Pharmacological Studies on Alkylaminoheptane Derivatives. I: Tracheal Muscle Spasmolytic Action of N-Alkyl-1, 5-dimethylhexylamine Derivatives". Yakugaku Zasshi. 80 (9): 1153–1155. doi:10.1248/yakushi1947.80.9_1153. ISSN 0031-6903.
- ^ a b c d e Ota Y, Enomoto R, Ishiguro Y (1960). "Pharmacological Studies on Alkylaminoheptane Derivatives. II: Pharmacological Action of N-Isopropyl-1, 5-dimethylhexylamine Hydrochloride. (1)". Yakugaku Zasshi. 80 (9): 1156–1159. doi:10.1248/yakushi1947.80.9_1156. ISSN 0031-6903.
- ^ a b c Ota Y, Fuchibe K, Takahashi M (1961). "Pharmacological Studies on Alkylaminoheptane Derivatives. III: Pharmacological Action of N-Isopropyl-1, 5-dimethylhexylamine Hydrochloride. (2)". Yakugaku Zasshi. 81 (3): 394–402. doi:10.1248/yakushi1947.81.3_394. ISSN 0031-6903.
- ^ a b c Ota Y (1961). "Pharmacological Studies on Alkylaminoheptane Derivatives. IV: Blood Pressor, Antispasmodic and Capillary Permeability Inhibiting Action of N-Alkyl-1, 5-dimethylhexylamine Derivatives". Yakugaku Zasshi. 81 (3): 403–407. doi:10.1248/yakushi1947.81.3_403. ISSN 0031-6903.
- ^ a b c Ota Y, Watabe M, Takahashi M (1961). "Pharmacological Studies on Alkylaminoheptane Derivatives. V: Pharmacological Action of N-isopropyl-1, 5-dimethylhexylamine Hydrochloride. (3)". Yakugaku Zasshi. 81 (3): 407–414. doi:10.1248/yakushi1947.81.3_407. ISSN 0031-6903.
- ^ a b c Ota Y (1961). "Pharmacological Studies on Alkylaminoheptane Derivatives. VI: Pharmacological Action of N-Isopropyl-1, 5-dimethyl-hexylamine Hydrochloride. (4)". Yakugaku Zasshi. 81 (3): 415–420. doi:10.1248/yakushi1947.81.3_415. ISSN 0031-6903.
- ^ Small C, Cheng MH, Belay SS, Bulloch SL, Zimmerman B, Sorkin A, et al. (August 2023). "The Alkylamine Stimulant 1,3-Dimethylamylamine Exhibits Substrate-Like Regulation of Dopamine Transporter Function and Localization". J Pharmacol Exp Ther. 386 (2): 266–273. doi:10.1124/jpet.122.001573. PMC 10353075. PMID 37348963.
- ^ Docherty JR (June 2008). "Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA)". Br J Pharmacol. 154 (3): 606–622. doi:10.1038/bjp.2008.124. PMC 2439527. PMID 18500382.
- ^ Delicado EG, Fideu MD, Miras-Portugal MT, Pourrias B, Aunis D (August 1990). "Effect of tuamine, heptaminol and two analogues on uptake and release of catecholamines in cultured chromaffin cells". Biochem Pharmacol. 40 (4): 821–825. doi:10.1016/0006-2952(90)90322-c. PMID 2386550.
- ^ Alsufyani HA, Docherty JR (January 2019). "Methylhexaneamine causes tachycardia and pressor responses indirectly by releasing noradrenaline in the rat". Eur J Pharmacol. 843: 121–125. doi:10.1016/j.ejphar.2018.10.047. PMID 30395850.
- ^ Schlessinger A, Geier E, Fan H, Irwin JJ, Shoichet BK, Giacomini KM, et al. (September 2011). "Structure-based discovery of prescription drugs that interact with the norepinephrine transporter, NET". Proc Natl Acad Sci U S A. 108 (38): 15810–15815. doi:10.1073/pnas.1106030108. PMC 3179104. PMID 21885739.
- ^ Rickli A, Hoener MC, Liechti ME (September 2019). "Pharmacological profiles of compounds in preworkout supplements ("boosters")". Eur J Pharmacol. 859: 172515. doi:10.1016/j.ejphar.2019.172515. PMID 31265842.
- ^ a b "Iproheptine". PubChem. Retrieved 8 December 2024.
