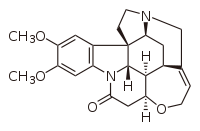
Brucine
| |
| Names | |
|---|---|
| IUPAC name
2,3-Dimethoxystrychnidin-10-one
| |
| Systematic IUPAC name
(4bR,4b1S,7aS,8aR,8a1R,12aS)-2,3-Dimethoxy-4b1,5,6,7a,8,8a,8a1,11,12a,13-decahydro-14H-12-oxa-7,14a-diaza-7,9-methanocyclohepta[cd]cyclopenta[g]fluoranthen-14-one | |
| Other names
2,3-Dimethoxystrychnine
10,11-Dimethoxystrychnine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.014 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1570 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C23H26N2O4 | |
| Molar mass | 394.471 g·mol−1 |
| Melting point | 178 °C (352 °F; 451 K) |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H300, H330, H412 | |
| P260, P264, P270, P271, P273, P284, P301+P310, P304+P340, P310, P320, P321, P330, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Brucine is an alkaloid closely related to strychnine, most commonly found in the Strychnos nux-vomica tree. Brucine poisoning is rare, since it is usually ingested with strychnine, and strychnine is more toxic than brucine. In chemical synthesis, it can be used as a tool for stereospecific chemical syntheses.
Brucine's name derives from this of the genus Brucea, named after James Bruce who brought back Brucea antidysenterica from Ethiopia.
History
[edit]Brucine was discovered in 1819 by the French chemist Pelletier and the French pharmacist Caventou in the bark of the Strychnos nux-vomica tree.[1] While its chemical structure was not deduced until much later, it was determined that it was closely related to strychnine in 1884 when the chemist Hanssen converted both strychnine and brucine into the same molecule.[2]
Identification
[edit]Brucine can be detected and quantified using liquid chromatography-mass spectrometry.[3] Historically, brucine was distinguished from strychnine by its reactivity toward chromic acid.[4]
Applications
[edit]Chemical applications
[edit]Since brucine is a large chiral molecule, it has been used in chiral resolution. Fisher first reported its use as a resolving agent in 1899, and it was the first natural product used as an organocatalyst in a reaction resulting in an enantiomeric enrichment by Marckwald, in 1904.[5] Its bromide salt has been used as the stationary phase in HPLC to selectively bind one of two anionic enantiomers.[6] Brucine has also been used for fractional crystallization in acetone to resolve dihydroxy fatty acids,[7] as well as diarylcarbinols.[8]
Medical applications
[edit]While brucine has been shown to have good anti-tumor effects, on both hepatocellular carcinoma[9] and breast cancer,[10] its narrow therapeutic window has limited its use as a treatment for cancer.
Brucine is also used in traditional Chinese medicine as an anti-inflammatory and analgesic agent,[11] as well as in some Ayurveda and homeopathy drugs.[12]
Alcohol denaturant
[edit]Brucine is one of the many chemicals used as a denaturant to make alcohol unfit for human consumption.[13]
Cultural references
[edit]One of the most famous cultural references to brucine occurs in The Count of Monte Cristo, the novel by French author Alexandre Dumas. In a discussion of mithridatism, Monte Cristo states:
“Well, suppose, then, that this poison was brucine, and you were to take a milligramme the first day, two milligrams the second day, and so on…at the end of a month, when drinking water from the same carafe, you would kill the person who drank with you, without your perceiving…that there was any poisonous substance mingled with this water.”[14]
Brucine is also mentioned in the 1972 version of The Mechanic, in which the hitman Steve McKenna betrays his mentor, ageing hitman Arthur Bishop, using a celebratory glass of wine spiked with brucine, leaving Bishop to die of an apparent heart attack.[15]
Such fictions run contrary to reality in the very properties which make brucine useful as a denaturant, and useless as a covert poison. While being only about one-eighth as toxic as strychnine, its threshold of bitterness occurs at 69 % greater dilution. A drink laden with brucine, overwhelmingly bitter at far below lethal concentration, would cause an intended victim to gag on the first sip.
Safety
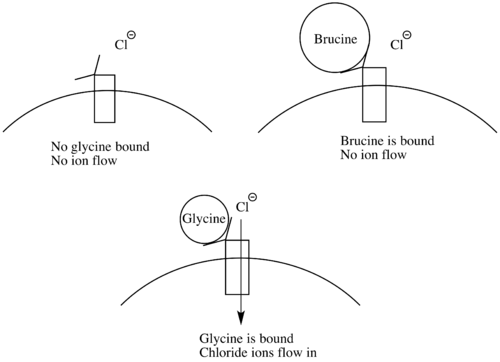
[edit]Brucine intoxication occurs very rarely, since it is usually ingested with strychnine. Symptoms of brucine intoxication include muscle spasms, convulsions, rhabdomyolysis, and acute kidney injury. Brucine’s mechanism of action closely resembles that of strychnine. It acts as an antagonist at glycine receptors and paralyzes inhibitory neurons.
The probable lethal dose of brucine in adults is 1 g.[16] In other animals, the LD50 varies considerably.
| Animal | Route of entry | LD50[17] |
|---|---|---|
| Mouse | Subcutaneous | 60 mg/kg |
| Rat | Intraperitoneal | 91 mg/kg |
| Rabbit | Oral | 4 mg/kg |
References
[edit]- ^ Wormley, T (1869). Micro-chemistry of poisons including their physiological, pathological, and legal relations: Adapted to the use of the medical jurist, physician, and general chemist. New York: W. Wood.
- ^ Buckingham, J (2007). Bitter Nemesis: The Intimate History of Strychnine. CRC Press. p. 225.
- ^ Teske, J; Weller, J; Albrecht, U; Fieguth, A (2011). "Fatal Intoxication Due to Brucine". Journal of Analytical Toxicology. 35 (4): 248–253. doi:10.1093/anatox/35.4.248. PMID 21513620.
- ^ Glasby, J. (1975). Encyclopedia of the alkaloids. New York: Plenum Press. p. 214. ISBN 9780306308451.
- ^ Koskinen, A (1993). Asymmetric synthesis of natural products. Chichester: J. Wiley. pp. 17, 28–29.
- ^ Zarbua, K; Kral, V (2002). "Quaternized brucine as a novel chiral selector". Tetrahedron: Asymmetry. 13 (23): 2567–2570. doi:10.1016/s0957-4166(02)00715-2.
- ^ Malkar, N; Kumar, V (1998). "Optical resolution of (±)-Threo-9,10,16-trihydroxy hexadecanoic acid using (−)brucine". Journal of the American Oil Chemists' Society. 75 (10): 1461–1463. doi:10.1007/s11746-998-0202-9. S2CID 83662051.
- ^ Toda, F; Tanaka, K; Koshiro, K (1991). "A New Preparative Method for Optically Active Diarylcarbinols". Tetrahedron: Asymmetry. 2 (9): 873–874. doi:10.1016/s0957-4166(00)82198-9.
- ^ Qin, J (2012). "Anti-Tumor Effects of Brucine Immune-Nanoparticles on Hepatocellular Carcinoma". International Journal of Nanomedicine. 7: 369–379. doi:10.2147/IJN.S27226. PMC 3273973. PMID 22334771.
- ^ Serasanambati, M; Chilakapati, S; Vanagavaragu, J; Cilakapati, D (2014). "Inhibitory effect of gemcitabine and brucine on MDA MB-231 human breast cancer cells". International Journal of Drug Delivery. 6. Archived from the original on 2016-03-04. Retrieved 2015-05-02.
- ^ Zhang, J; Wang, S; Chen, X; Zhide, H; Xiao, M (2003). "Capillary Electrophorese with Field-Enhanced Stacking for Rapid and Sensitive Determination of Strychnine and Brucine". Analytical and Bioanalytical Chemistry. 376 (2): 210–213. doi:10.1007/s00216-003-1852-y. PMID 12692702. S2CID 7832819.
- ^ Rathi, A; Srivastava, N; Khatoon, S; Rawat, A (2008). "TLC Determination of Strychnine and Brucine of Strychnos nun vomica in Ayurveda and Homeopathy Drugs". Chromatographia. 67 (7–8): 607–613. doi:10.1365/s10337-008-0556-z. S2CID 94626190.
- ^ "List of denaturants authorized for denatured spirits". www.law.cornell.edu. Cornell Law School. 30 August 2016. Retrieved 2019-08-24.
- ^ Dumas, Alexandre (1845). The Count of Monte Cristo. Feedbooks. p. 622.
- ^ "Synopsis for The Mechanic". IMDb. Retrieved 30 April 2015.
- ^ Gosselin, R. E.; Smith, R. P.; Hodge, H. C. (1984). Clinical Toxicology of Commercial Products (5 ed.). Baltimore/London: Williams & Wilkins.
- ^ "Brucine". Toxnet. NIH. Retrieved 2018-10-07.
External links
[edit]- Brucine, inchem.org
