Atomic orbital model: Difference between revisions
Phil Bridger (talk | contribs) |
This is an original work. Reproduction without permission is illegal. |
||
| Line 1: | Line 1: | ||
{{unreferenced|date=June 2008}} |
{{unreferenced|date=June 2008}} |
||
The '''Atomic Orbital Model''' is the currently accepted model of the [[electron]]s in an [[atom]]. It is sometimes called the '''Wave Mechanics Model'''. In the atomic orbital model, the atom consists of a [[atomic nucleus|nucleus]] surrounded by orbiting electrons. These electrons exist in [[atomic orbital]]s, which are a set of [[quantum]] states of the negatively charged electrons trapped in the [[electrical field]] generated by the positively charged nucleus. Classically, the orbits can be likened to the planets orbiting the sun. However, the atomic orbital model can only be described by [[quantum mechanics]], in which case the electrons are more accurately described as standing waves surrounding the nucleus. |
|||
==Timeline of development== |
==Timeline of development== |
||
The developments of the orbital model proceeded along the following timeline: |
The developments of the orbital model proceeded along the following timeline: |
||
5th century BC: The ancient Greek philosophers [[Leucippus]] and his pupil [[Democritus]] proposed that all matter was composed of small indivisible particles called atoms. |
|||
1800-1810: [[John Dalton|Dalton]] examined the empirical compositions of chemical compounds and proposed a set of rules regarding the properties of the [[Chemical element|elements]] and how they combined to form compounds, leading to the Billiard Ball theory. |
1800-1810: [[John Dalton|Dalton]] examined the empirical compositions of chemical compounds and proposed a set of rules regarding the properties of the [[Chemical element|elements]] and how they combined to form compounds, leading to the Billiard Ball theory. |
||
| ⚫ | |||
1897: [[J. J. Thompson]] published his work on the discovery of the electron. This led to the [[plum pudding model]] of the atom. |
|||
| ⚫ | |||
1909: [[Ernest Rutherford]] led scattering experiments on [[gold]] foils (gold-foil experiment), and thereby discovered the nucleus |
1909: [[Ernest Rutherford]] led scattering experiments on [[gold]] foils (gold-foil experiment), and thereby discovered the nucleus |
||
1911: Rutherford proposed the [[Rutherford model]] based on the earlier experimental work in which a cloud of electrons orbit the nucleus. |
|||
1913: [[Niels Bohr]] proposed the [[Planetary Model]] of the atom. |
1913: [[Niels Bohr]] proposed the [[Planetary Model]] of the atom. |
||
| Line 26: | Line 18: | ||
{{main|Atomic Theory}} |
{{main|Atomic Theory}} |
||
after the Greek word for unity. |
|||
The theory of the atom proposed by the ancient Greeks can be summed up in a single thought experiment. |
|||
Suppose we take a solid object, and divide that object into two. Now we repeat the process over and over again, continually dividing the remaining piece into two. Will we be able to continue dividing the object indefinitely, or will we come to a point where we find a smallest indivisible particle? |
|||
This led to a school of thought that believed that there was a smallest indivisible unit, and this unit was called the [[atom]] after the Greek word for unity. Adherents to this philosophy were called [[atomists]]. |
|||
=== Dalton's model === |
=== Dalton's model === |
||
| Line 57: | Line 45: | ||
[[Image:Stylised Lithium Atom.svg|thumb|A stylised Lithium atom.]] |
[[Image:Stylised Lithium Atom.svg|thumb|A stylised Lithium atom.]] |
||
In 1909, Ernest Rutherford (with [[Hans Geiger]] and [[Ernest Marsden]]) performed an [[Geiger-Marsden experiment|experiment]], which consisted of firing alpha particles into a thin gold foil and measuring the scattering angles of those particles. The results showed that majority of the atom consisted of empty space. In 1911, Rutherford proposed a model to explain the experimental results in which the atom was made up of a nucleus of approximately 10<sup>-15</sup> m in diameter, surrounded by an electron cloud of approximately 10<sup>-10</sup> m in diameter. |
|||
Following this discovery, the study of the atom split into two distinct fields, [[nuclear physics]], which studies the properties and structure of the nucleus of atoms, and [[atomic physics]], which examines the properties of the electrons surrounding the nucleus. |
|||
The electrons in the Rutherford model were thought to orbit the nucleus much like the planets orbit the sun. However, this model suffered from a number of problems. |
The electrons in the Rutherford model were thought to orbit the nucleus much like the planets orbit the sun. However, this model suffered from a number of problems. |
||
The first is that, unlike the planets orbiting the sun, the electrons are charged particles, and the motion of a charged particle is expected to produce electromagnetic radiation. As an orbiting electron produces electro-magnetic radiation, it loses energy, and would thus spiral into the nucleus. |
|||
The second problem was that there was no mechanism to stratify the radius of the orbits. Thus, even if the first problem could be overcome, the second problem meant that there should be a continuous range of electron orbitals available with a continuous range of energies. This in turn would predict that the emission / absorption spectra of atoms would be continuous distributions rather than the highly-peaked line spectra that were observed. (The discovery of the photo-electric effect by Einstein had shown that the frequency of electromagnetic energy was proportional to the energy, and thus that each line in the line spectra corresponded to a very well-defined difference in energy between separate atomic orbitals around the same nucleus). |
|||
=== Bohr atomic model === |
=== Bohr atomic model === |
||
| Line 72: | Line 53: | ||
[[Image:Bohr-atom-PAR.svg|thumb|The Rutherford Bohr model of the hydrogen atom.]] |
[[Image:Bohr-atom-PAR.svg|thumb|The Rutherford Bohr model of the hydrogen atom.]] |
||
After the discovery of the photoelectric effect, the connection between the structure of electrons in atoms and the emission and absorption spectra of atoms became an increasingly useful tool in the understanding of electrons in atoms. The most prominent feature of emission and absorption spectra was that these spectra contained discrete lines. The significance of the Bohr model was that it related the lines in emission and absorption spectra to the energy differences between the orbits that electrons could take around an atom. This was achieved by giving the electrons some kind of wave-like properties. In particular, electrons were assumed to have a wavelength (a property that had previously been discovered, but not entirely understood). The Bohr model was therefore not only a significant step towards the understanding of electrons in atoms, but also a significant step towards the development of the wave/particle duality of quantum mechanics. |
|||
The premise of the Bohr model was that electrons had a wavelength, which was a function of its momentum, and therefore an orbiting electron would need to orbit at a multiple of the wavelength. The Bohr model was thus a classical model with an additional constraint provided by the 'wavelength' argument. In our current understanding of physics, this 'wavelength' argument is known to be an element of quantum mechanics, and for that reason the Bohr model is called a semi-classical model. |
The premise of the Bohr model was that electrons had a wavelength, which was a function of its momentum, and therefore an orbiting electron would need to orbit at a multiple of the wavelength. The Bohr model was thus a classical model with an additional constraint provided by the 'wavelength' argument. In our current understanding of physics, this 'wavelength' argument is known to be an element of quantum mechanics, and for that reason the Bohr model is called a semi-classical model. |
||
| Line 79: | Line 59: | ||
== Current theory == |
== Current theory == |
||
With the development of quantum mechanics, it was found that the electrons orbiting a nucleus could not be fully described as particles, but needed to be explained by the wave-particle duality. In this sense, the electrons have the following properties: |
|||
=== Wave-like properties === |
|||
The electrons do not orbit the nucleus in the sense of a planet orbiting the sun, but instead exist as standing waves. The lowest possible energy an electron can take is therefore analogous to the fundamental frequency of a wave on a string. Higher energy states are then similar to harmonics of the fundamental frequency. |
|||
The electrons are never in a single point location, although the probability of interacting with the electron at a single point can be found from the wavefunction of the electron |
|||
=== Particle-like properties === |
|||
There is always an integer number of electrons orbiting the nucleus. |
|||
Electrons jump between orbitals in a particle-like fashion. For example, if a single photon strikes the electrons, only a single electron changes states in response to the photon. |
|||
The electrons retain particle like-properties such as: each wave state has the same electrical charge as the electron particle. Each wave state has a single discrete spin (spin up or spin down). |
|||
=== Describing the electrons === |
|||
Because the electrons around a nucleus exist as a wave-particle duality, they cannot be described by a location and momentum. Instead, they are described by a set of quantum numbers that encompasses both the particle-like nature and the wave-like nature of the electrons. Each set of quantum numbers corresponds to a wavefunction. The quantum numbers are: |
|||
The [[principal quantum number]], n, is analogous to the harmonic of the electrons. That is, the n=1 states are analogous to the fundamental frequency of a wave on a string, and the n=2 states are analogous to the first harmonic, etc. |
|||
The [[azimuthal quantum number]], l, describes the orbital angular momentum of each electron. Note that this has no classical analog. The number l is an integer between 0 and (n - 1). |
|||
The [[magnetic quantum number]], m<sub>l</sub>, describes the magnetic moment of an electron in an arbitrary direction. The number m<sub>l</sub> is an integer between -l and l. |
|||
The [[spin quantum number]], s, describes the spin of each electron (spin up or spin down). The number s can be +{{frac|1|2}} or -{{frac|1|2}}. |
|||
These quantum numbers can only be determined by a full quantum mechanical analysis of the atom. There is no way to describe them using classical physical principles. A more technical analysis of these quantum numbers and how they are derived is given in the [[atomic orbital]] article. |
|||
Furthermore, the [[Pauli Exclusion Principle]] states that no two electrons can occupy the same quantum state. That is, every electron that is orbiting the same nucleus must have a unique combination of quantum numbers. |
|||
=== Predictions of the model === |
|||
Under quantum mechanics, each quantum state has a well-defined energy. When applied to atomic orbitals, this means that each state has a specific energy, and that if an electron is to move between states, the energy difference is also very fixed. |
|||
Consider two states of the Hydrogen atom: |
|||
State 1) n=1, l=0, m<sub>l</sub>=0 and s=+{{frac|1|2}} |
|||
State 2) n=2, l=0, m<sub>l</sub>=0 and s=+{{frac|1|2}} |
|||
By quantum theory, state 1 has a fixed energy of E<sub>1</sub>, and state 2 has a fixed energy of E<sub>2</sub>. Now, what would happen if an electron in state 1 were to move to state 2? For this to happen, the electron would need to gain an energy of exactly E<sub>2</sub> - E<sub>1</sub>. If the electron receives energy that is less than or greater than this value, it cannot jump from state 1 to state 2. Now, suppose we irradiate the atom with a broad-spectrum of light. Photons that reach the atom that have an energy of exactly E<sub>2</sub> - E<sub>1</sub> will be absorbed by the electron in state 1, and that electron will jump to state 2. However, photons that are greater or lower in energy cannot be absorbed by the electron, because the electron can only jump to one of the orbitals, it cannot jump to a state between orbitals. The result is that only photons of a specific frequency will be absorbed by the atom. This creates a line in the spectrum, known as an absorption line, which corresponds to the energy difference between states 1 and 2. |
|||
The atomic orbital model thus predicts line spectra, which are observed experimentally. This is one of the main validations of the atomic orbital model. |
|||
== Further reading == |
|||
The details of how atomic orbitals are characterized, and approximations used to calculate them are described in the article [[atomic orbital]]. |
|||
Details on the structure of electrons in compounds can be found in [[molecular orbital]]. |
|||
The study of how chemical bonds are formed by orbital electrons to form molecules is discussed in [[quantum chemistry]]. |
|||
The study of electrons in crystalline materials is a topic of [[solid state physics]] and [[condensed matter physics]]. |
|||
{{Atomic models}} |
{{Atomic models}} |
||
Revision as of 21:49, 20 October 2008
Timeline of development
The developments of the orbital model proceeded along the following timeline:
1800-1810: Dalton examined the empirical compositions of chemical compounds and proposed a set of rules regarding the properties of the elements and how they combined to form compounds, leading to the Billiard Ball theory.
This discovery would later be used to relate emission and absorption spectra to the electron structure of atoms, leading to the equation E=MC2
1909: Ernest Rutherford led scattering experiments on gold foils (gold-foil experiment), and thereby discovered the nucleus
1913: Niels Bohr proposed the Planetary Model of the atom.
1925: Erwin Schrödinger proposed the Schrödinger equation, which allowed the electrons in an atom to be analyzed quantum mechanically (Quantum Mechanics). This led to the current atomic orbital model of the atom, the Quantum Mechanic model or the Electron Cloud model
Ancient Greeks
after the Greek word for unity.
Dalton's model
The chemist John Dalton examined the empirical (derived from or guided by experience or experiment) proportions of elements that made up chemical compounds.
This marked the first truly scientific theory of the atom, since Dalton reached his conclusions by experimentation and examination of the results in an empirical fashion.
At this stage, the atom was still seen as an indivisible object, with no internal structures.
This model is consistent with the concept of an ideal gas as being made up of molecules that exert negligible forces on one another and whose volume is negligible relative to the volume occupied by the gas. This model assumes that each mixture component behaves as an ideal gas as if it were alone at a given specific temperature and volume of the mixture
As the atoms do themselves
Plum pudding model

The discovery of the electron by J. J. Thomson showed that atoms did indeed have some kind of internal structure. The plum pudding model of the atom described the atom as a "pudding" of positive charge, with negatively charged electrons embedded in this pudding like plums in a plum pudding.
Rutherford model
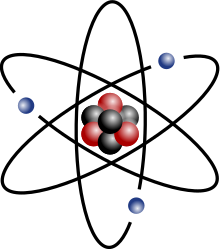
The electrons in the Rutherford model were thought to orbit the nucleus much like the planets orbit the sun. However, this model suffered from a number of problems.
Bohr atomic model

The premise of the Bohr model was that electrons had a wavelength, which was a function of its momentum, and therefore an orbiting electron would need to orbit at a multiple of the wavelength. The Bohr model was thus a classical model with an additional constraint provided by the 'wavelength' argument. In our current understanding of physics, this 'wavelength' argument is known to be an element of quantum mechanics, and for that reason the Bohr model is called a semi-classical model.
The Bohr model was able to explain the emission and absorption spectra of Hydrogen. The energies of electrons in the n=1, 2, 3, etc. states in the Bohr model match those of current physics. However, this did not explain similarities between different atoms, as expressed by the periodic table, such as why Helium (2 electrons), Neon (8 electrons), and Argon (18 electrons) exhibits similar chemical behaviour. Modern physics explains this by noting that the n=1 state can hold 2 electrons, the n=2 state can hold 6 electrons, and the n=3 state can hold 10 electrons. In the end, this was solved by the discovery of modern quantum mechanics and the Pauli Exclusion Principle.
